SUMMARY:
Meningiomas, the most common primary intracranial neoplasms, account for more than one-third of primary CNS tumors. While traditionally viewed as benign, meningiomas can be associated with considerable morbidity, and specific meningioma subgroups display more aggressive behavior with higher recurrence rates. The risk stratification for recurrence has been primarily associated with the World Health Organization (WHO) histopathologic grade and extent of resection. However, a growing body of literature has highlighted the value of molecular characteristics in assessing recurrence risk. While maintaining the previous classification system, the 5th edition of the 2021 WHO Classification of Central Nervous System tumors (CNS5) book expands upon the molecular information in meningiomas to help guide management. The WHO CNS5 stratifies meningioma into 3 grades (1−3) based on histopathology criteria and molecular profile. The telomerase reverse transcriptase promoter mutations and cyclin-dependent kinase inhibitor 2A/B (CDKN2A/B) deletions now signify a grade 3 meningioma with increased recurrence risk. Tumor location also correlates with underlying mutations. Cerebral convexity and most spinal meningiomas carry a 22q deletion and/or NF2 mutations, while skull base meningiomas have AKT1, TRAF7, SMO, and/or PIK3CA mutations. MRI is the primary imaging technique for diagnosing and treatment-planning of meningiomas, while DOTATATE PET imaging offers supplementary information beyond anatomic imaging. Herein, we review the evolving molecular landscape of meningiomas, emphasizing imaging/genetic biomarkers and treatment strategies relevant to neuroradiologists.
ABBREVIATIONS:
- AKT1
- AKT serine/threonine kinase 1
- BAP1
- BRCA1-associated protein 1
- CDK4/6
- cyclin-dependent kinases 4 and 6
- CDKN2A/B
- cyclin-dependent kinase inhibitor 2A/B
- CNS5
- Classification of Central Nervous System Tumors, fifth edition
- KLF4
- Krüppel-like factor 4
- mTOR
- mammalian target of rapamycin
- NF2
- neurofibromatosis type 2
- PIK3CA
- phosphatidylinositol-4,5-Bisphosphate 3-Kinase catalytic subunit alpha
- POLR2A
- RNA polymerase II subunit A
- pTERT
- telomerase reverse transcriptase promoter
- SMARCB1
- SWItch/sucrose non-fermentable related, matrix associated, actin dependent regulator of chromatin, subfamily b, member 1
- SMO
- smoothened, frizzled class receptor
- SM
- spinal meningioma
- SUVmax
- maximum standard uptake value
- TERT
- telomerase reverse transcriptase
- TRAF7
- tumor necrosis factor receptor–associated factor 7
- WHO
- World Health Organization
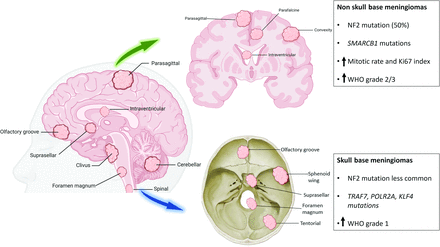
Meningiomas are the most common primary intracranial extra-axial tumor, representing 37.6% of all intracranial tumors in adults. The annual age-adjusted rate is 8.58 cases per 100,000 in the United States.1 Most (80.3%) meningiomas are located in the cerebral meninges, 4.2% in the spinal meninges, and approximately 14.7% lack a specified meningeal site.1 Meningiomas are most frequently diagnosed between 40 and 70 years of age, with an age peak at around 65 years. Women are 2.3 times more likely to have benign (grade 1) meningiomas than men.1 Spinal meningiomas (SMs) account for 25%–46% of all primary spinal tumors.2 The SM incidence ranges between 0.193 and 0.33 cases/100,000 persons.3 They have a strong female predilection (75%–90%), with the peak incidence after 6 decades.2 The fifth edition of World Health Organization (WHO) Classification of Central Nervous System Tumors (CNS5 hereafter) incorporates molecular information to categorize meningiomas into 3 grades: benign (grade 1), atypical (grade 2), and anaplastic (or malignant, grade 3), applying atypical and anaplastic criteria to each subtype. CNS5 has recommended using an Arabic numeral grading system (1−3).4⇓–6 Meningiomas originate from the arachnoid cells of the dura. They are generally benign, incidentally diagnosed, extra-axial dural-based enhancing masses at cerebral convexities, para-/suprasellar regions, tentorium, and occasionally intraventricular (Fig 1). Larger, symptomatic lesions present with mass effects, seizures, or increased intracranial pressure.7⇓⇓–10 SMs are commonly located in the thoracic (60–70%), followed by the cervical (20–30%) and lumbar region (5–10%).2,11,12 They present as gradual localized back pain, sometimes radiating to the extremities. Cord or nerve root compression may lead to weakness, numbness, and tingling.11.
Illustration depicting the common location of meningiomas and associated genetic/cytogenetic alteration and grades. NF2 and SMARCB1 mutations are more frequently seen in convexity meningiomas. Grade 2 and 3 meningiomas are more common along the convexity than the skull base. AKT1, KLF4, TRAF7, and POLR2A genetic changes are more frequently seen in skull base meningiomas. Grade 1 is more common in the posterior fossa. Grade 2 (atypical) meningiomas are more frequently seen along the brain convexity and spine and can have a loss of a copy of chromosomes 1, 10, or 14.
Meningiomas are associated with genetic syndromes, including most frequent Neurofibromatosis type 2 (NF2), and rare syndromes such as Gorlin, Li Fraumeni, NF1, and Von Hippel-Lindau. Mutations in the SMARCE1, SWItch/sucrose non-fermentable related, matrix associated, actin dependent regulator of chromatin, subfamily b, member 1 (SMARCB1), BRCA1-associated protein 1 (BAP1), SUFU, PTEN, and CREBBP genes are linked to various syndromes that increase individual sensitivity to radiation.13 The tumor location correlates with mutation spectra, with 22q deletion and/or NF2 mutations common in the convexity and SMs. Skull base meningiomas typically have AKT serine/threonine kinase 1 (AKT1), tumor necrosis factor receptor-associated factor 7 (TRAF7), smoothened, frizzled class receptor (SMO), and phosphatidylinositol-4,5-Bisphosphate 3-Kinase catalytic subunit alpha (PIK3CA) mutations (Fig 2).14 High-grade meningiomas predominantly originate from the convexity and non-skull base areas (Fig 3).15⇓⇓⇓⇓–20 Grade 1 meningiomas displace the brain and are easily separable, while higher grades are invasive, adhering to dural sinuses, skull, scalp, and skin.9,21 Extracranial metastases to the lung, pleura, bone, or liver are sporadic (0.67%) and are more common with grade 2 (2%) and 3 (9%) meningiomas.22 The incidence of WHO grade 1, 2, and 3 meningioma is 80.5%, 17.7%, and 1.7% respectively.1
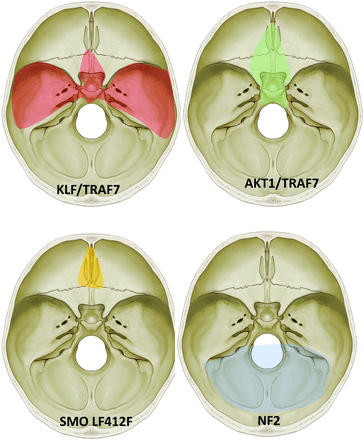
“Mutation map” of skull base meningiomas with a regional propensity of meningiomas dependent on specific mutations. Meningiomas arising from the sphenoid wing have KLF4/TRAF7 mutations, midline tumors have AKT1/TRAF7 mutations, and tumors originating from the olfactory groove tend to have SMO mutations. Meningiomas along the posterior skull base commonly have a loss of chromosome 22-loss (NF2). Adapted from Baranoski J. Smarcb1-Mutant Intracranial Meningiomas: A Distinct Subtype of Nf2-Mutant Tumors, 2015. Yale Medicine Thesis Digital Library. 1947.
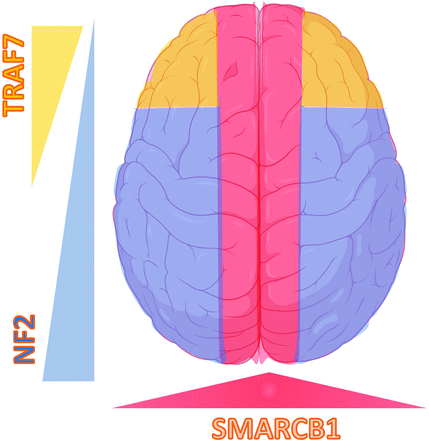
“Mutation map” of convexity meningiomas with the regional propensity of meningiomas dependent on a specific mutation. Meningiomas along the falx and midline parasagittal region tend to have the SMARCB1 mutation. Meningiomas along the posterior cerebral convexity tend to have NF2/chromosomal 22 with higher chances of TRAF mutation along the anterior cerebral convexity. Adapted from Baranoski J, Smarcb1-Mutant Intracranial Meningiomas: A Distinct Subtype of Nf2-Mutant Tumors, 2015. Yale Medicine Thesis Digital Library. 1947.
Management depends on tumor location, grade, and symptoms. Gross total resection is the primary treatment for most symptomatic and grade 1 meningiomas, and adjuvant radiation therapy is performed for grade 2 or 3.23 Incomplete resection or aggressive histopathologies are associated with recurrence and transformation into a higher grades.1,7,24 Benign meningiomas have excellent 10-year survival (83.7%), with better outcomes in the young. Malignant meningioma has a poor outcome (61.7% 10-year survival).1 Recurrences are common in grades 2 (50%) and 3 (90%).7
DISCUSSION
Updates in Recent WHO Guidelines
The CNS5 classification integrates molecular biomarkers for grading, which can supersede histologic features (Fig 4).25 Grade 3 criteria are based on the telomerase reverse transcriptase promoter (pTERT) mutation and homozygous deletion of cyclin-dependent kinase inhibitor 2A/B (CDKN2A/B) with a high mitotic rate (≥20/10 high power fields) and anaplastic histopathologic features (sarcoma/carcinoma/melanoma-like morphology) irrespective of histotype. Atypical or anaplastic meningiomas are defined across all histologic subtypes. Choroid and clear cell meningiomas are designated grade 2 due to a higher recurrence rate. Similarly, papillary and rhabdoid meningiomas can be grade 1, 2, or 3 and should not be graded on the basis of histology alone. CNS5 classification no longer uses other “atypical” features to designate grade 2 for other morphologic subtypes. The term “anaplastic” is replaced by grading based on molecular features that can categorize a tumor as grade 3, even without evident anaplastic histology characteristics.6 Rhabdoid and papillary variants may not meet CNS5 anaplastic grading without high-grade features, despite some studies cautioning against grading them similar to non-rhabdoid tumors due to potential aggressive behavior.26 The introduction of genome-wide DNA methylation arrays has further refined classification.27 A molecular-morphologic integrated score allocates points to the histologic grade, epigenetic methylation family, and specific copy-number variations. It is more accurate in the prognostic stratification of meningiomas.28
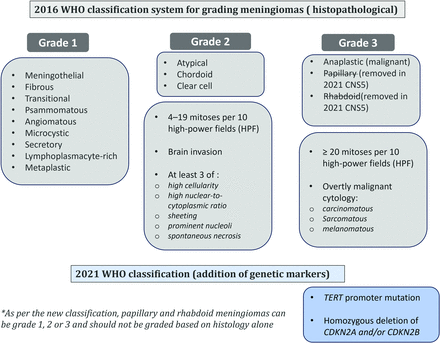
Chart illustration of the grading of meningiomas according to the 2016 WHO classification scheme and the changes made in the 2021 CNS5 classification, with the CNS5 addition of new molecular entities for grading and removal of a few grade 3 histologic subtypes.
Histopathological grading and its limitation
Meningiomas are characterized histopathologically by whorls of tumor cells, nuclear pseudo-inclusions, pseudo-syncytial growth, and concentric calcifications, called “psammoma bodies.”29 Immunohistochemical markers, including epithelial membrane antigen, somatostatin receptor 2A (SSTR), progesterone (70–80%), and estrogen receptors (5–30%) aid in differentiating meningiomas from other dural-based lesions.30 Supplementary Table 1 provides essential and desirable diagnostic criteria for meningioma. Under the WHO 2016 classification, meningiomas were graded (I-III) based on the mitotic index, histologic features (sheeting, hypercellularity, prominent nucleoli, and necrosis) and specific histotype. Meningothelial morphology is the most common histologic subtype and are usually grade 1. Presence of 4–19 mitotic figures per 10 high-power fields (HPF) is grade 2, whereas 20 or more mitotic figures is a criterion for grade 3. Histologic features like sheeting of tumor cells, spontaneous necrosis and brain invasion are findings of grad 2 lesion (Fig 5).29 CNS5 outlines brain invasion criteria, necessitating tumors to breach the pia mater rather than merely indent the brain or extend along perivascular spaces.6 Brain invasion is regarded as an unfavorable feature in CNS5; however, it is not uniformly accepted.31 Apart from mitotic figures (20 or more/10 HPF), presence of overtly malignant cytology, like sarcomatous or melanomatous, is an independent criteria for grade 3 meningiomas (Fig 6). WHO grading predicted recurrence risk, but consistent grading reproducibility remained challenging, with 87.2% interobserver agreement in a multicenter trial. Grade II tumors exhibit a higher inter-observer discrepancy (12.2%) compared to Grade I (7%) and Grade III (6.4%) tumors.32 Besides, some Grade II tumors behave similarly to grade I or III, leading to unexpected outcomes highlighting limitations of classical histological grading.4,33 Clinical and radiological features inadequately distinguish grade I from atypical grade 2 meningiomas. Atypical meningiomas progress rapidly, display aggressive imaging, and tend to recur early.34 Differentiation between atypical and anaplastic meningioma is challenging due to the continuum of increasing anaplasia. Interobserver reproducibility is better for the mitotic count than for anaplasia.35
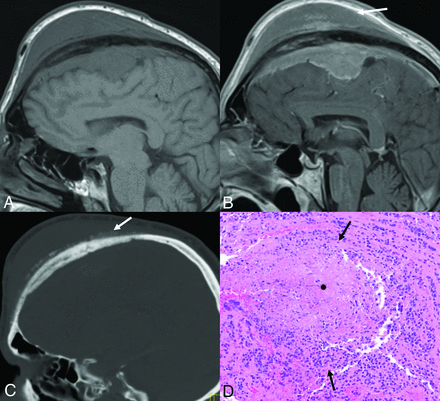
Transcalvarial frontal atypical, grade 2 meningioma in a 54-year-old man. Sagittal T1-weighted (A) and contrast-enhanced (B) images reveal a large frontal convexity meningioma with intraosseous components and transcalvarial extension into the scalp (B, arrow). Mild hyperostosis of the adjacent calvaria along with a “hair-on-end” periosteal reaction along the outer table is seen on CT (C, arrow). Tumor shows meningothelial morphology, with, however, other features such as focal spontaneous necrosis (D, asterisk) and peripheral nests of tumor cells (D, arrows). A high Ki-67 rate in hot spot areas and areas of tumor that are devoid of progesterone receptors is also noted, supportive of the diagnosis of atypical meningioma (WHO grade 2). Mitotic count of 4 mitoses per 10 high-power fields is noted, on its own fulfilling the criteria for a diagnosis of an atypical meningioma.
An anaplastic (malignant) grade 3 meningioma along the right middle cranial fossa floor. Coronal T2-weighted (A) and coronal and axial contrast-enhanced (B and C) images depict the meningioma with strongly suggestive imaging features of parenchymal invasion, including poor interface between the tumor and cortex (A, arrow) and nodules as foci of cortical enhancement (B and C, white arrows) along with linear leptomeningeal enhancement (B, black arrow). Histopathology shows frank anaplasia cells with numerous pleomorphic cells giving this tumor a sarcomatoid appearance (D, arrows) with a grade 3 classification. Mitotic count of 22 mitoses per 10 high-power fields was noted, on its own fulfilling the criteria for the diagnosis of an anaplastic meningioma.
Molecular and Genomic Characteristics in Meningiomas and Their Clinicopathologic Correlation
The clinicopathologic relevance of genetic alterations in meningiomas suggests certain alterations in specific subtypes and locations (Table). Additionally, higher-grade meningiomas exhibit more frequent abnormalities.8 NF2 alteration and/or 22 monosomy involves all grades and likely early tumor development events.36 NF2 alterations are prevalent in fibroblastic and transitional meningiomas (70%) and rare in meningothelial, secretory, and microcystic subtypes.37,38 Sporadic NF2 mutations are implicated in 40%–60% of meningiomas, while 50%–75% of those with germline mutations develop meningiomas.39,40 Many patients with NF2 have multiple meningiomas in addition to vestibular schwannomas (Online Supplemental Data).41 Meningiomas in patients with NF2 tend to have distinct clinical and genetic profiles compared with sporadic cases. Molecular research has identified additional meningioma mutations, including SMARCE1 (clear cell subtype), BAP1 (rhabdoid and papillary subtypes), Krüppel-like factor 4 (KLF4)/TRAF7 mutations, pTERT mutation, CDKN2A/B deletion, H3K27me3 loss, and methylome profiling.25 pTERT alterations increase telomerase reverse transcriptase (TERT) expression and telomere length as a diagnostic marker for WHO grade 3 meningioma.27,42,43 pTERT mutations have higher malignant transformation, early recurrence, and worse survival than their wild-type counterparts (2.7 versus 10.8 years).44 A meta-analysis (59 pTERT-mutated and 618 pTERT wild-type meningiomas) observed poor survival in pTERT-mutated meningiomas (58 versus 160 months).42
| Genetic Mutation Name | WHO Grade | Typical Location | Clinical Significance |
|---|---|---|---|
| NF2 | 1−3 | Parafalcine, posterior fossa–falcotentorial | Most common70% of fibroblastic and transitional meningiomasSporadic mutations present in 40%−60% of meningiomas50%−75% of patients with germline mutations develop meningiomasAssociated with larger, more aggressive course |
| TRAF7 | 1−3 | Central and lateral skull base | 2nd most commonSecretory subtypeHigh likelihood of hyperostosisMeningiomas tend to be benign, chromosome-stable |
| TERT | 3 | Any location | Commonly seen in higher grade, particularly grade 3Associated with shorter time to progression, shorter overall survival, and higher recurrence |
| CDKN2A/B | 3 | Any location | Mutations associated with shorter time to recurrenceClassification criteria for WHO grade 3 meningiomas |
| SMO/SUFU | 1 | Olfactory groove meningiomas, anterior skull base | Higher recurrence rates among olfactory groove meningiomasLarger tumor volume among anterior skull base meningiomasLinked to development of isolated familial and multiple meningiomas |
| KLF4 | 1 | Central and lateral skull base | Secretory subtypeLarger peritumoral brain edemaResults in up-regulation of HIF-1a pathwayMay respond to mTOR inhibition |
| POLR2A | 1 | Parasellar/tuberculum sellae | Found almost exclusively in WHO grade 1 meningiomas (meningothelial) |
| AKT1 | Anterior and middle skull base, posterior fossa | MeningothelialMutations occur with higher frequency among skull base meningiomasAssociated with shorter time to recurrence | |
| PIK3CA | 1–3 | Anterior and middle skull base | Low recurrence riskProgesterone and cyproterone antiandrogen therapy show higher PIK3CA mutation rates in skull base meningiomas |
| Hedgehog | Midline anterior skull base | Low-grade and less aggressive | |
| SMARCB1SMARCE1 | 1–3 | Parafalcine and lateral skull base | Clear cell typeSMARCB1 has been linked to multiple meningiomasSMARCE1 mutations linked with familial multiple spinal meningiomasHigher recurrence risk, faster growth |
| BAP1PBRM1 | 3 | Rhabdoid and papillary subtypesAggressive clinical behavior (consistent with CNS5 WHO grade 3) |
Note:— HIF-1-α indicates hypoxia-inducible factor 1.
Commonly identified germline and somatic mutations in meningiomas with corresponding WHO grade, location, and clinical significance
pTERT mutations may be acquired during lower-to-higher-grade progression. In a study of 40 patients, pTERT mutation was associated with higher recurrence (1.7; 95% CI, 0.65–4.44) and mortality (×2.5; 95% CI, 1.01–6.19) than pTERT wild-type (Fig 7).43 The CDKN2A/B tumor-suppressor gene deletion indicates aggressive grade 3 tumors.45,46 In a study of 528 patient with meningiomas, 4.9% showed CDKN2A/B deletions in grades 2 (27%) and 3 (73%), with a median progression time of 8 months. CDKN2A/B deletions showed worse outcomes even without pTERT mutations.46 The deletion of CDKN2A/B can be a crucial factor in upgrading the tumor from a histologic grade 1 to an anaplastic grade 3 (Fig 8).47 Cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors, upstream regulators of crucial cell cycle pathways, could be a potential target for systemic treatments of high-grade meningiomas.48 The PBRM1 mutation is common in papillary meningiomas.49 BAP1 mutations are present in approximately 10% of rhabdoid meningiomas and have an aggressive clinical behavior.6,50
Right intraorbital CNS WHO grade 3 meningioma with activation of the TERT promoter. A meningioma is initially classified as grade 2 (atypical) based on histopathologic features; however, it was upgraded to grade 3 on the basis of detection of TERT activation on chromosomal microarray. Preoperative contrast-enhanced MRI at presentation (A) and at 6 months’ follow-up (B) reveals rapid enlargement of the intraorbital meningioma (arrows). The patient had multiple postoperative recurrences during the next 18 months. Preoperative contrast-enhanced MRI (C and D) performed at 3-month intervals shows rapid enlargement of recurrent enhancing tumor along the orbital roof (C and D, arrows).
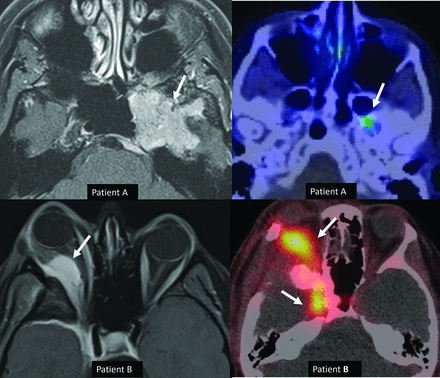
Histologic grade 1 skull base meningiomas in 2 different patients (patients A and B) with upgrading to grade 3 (anaplastic) in patient B based on CDKN2 deletion. Axial (A) contrast-enhanced MRI in patient A reveals a left middle cranial fossa meningioma (arrow) with no recurrence and minimal residual activity on the 1-year postresection follow-up DOTATATE scan (B, arrow). A right sphenoid wing meningioma with intraorbital extension is seen on axial contrast-enhanced MRI (C, arrow) in patient B. Tumor recurrence was noted at the 1-year postresection follow-up DOTATATE scan (D, arrows), consistent with high-grade morphology. Despite histologic grade 1, the meningioma was upgraded to grade 3 (anaplastic) on the basis of identification of a CDKN2 deletion.
Gene profiling is superior to routine histopathology in predicting recurrence risk. Four exclusive pathways drive meningioma development: heightened hedgehog signaling (SMO, SUFU, PRKAR1A), TRAF7, KLF4, and RNA polymerase II subunit A (POLR2A) mutations.18,51 In non-NF2-mutant meningiomas, AKT1, PIK3CA, TRAF7, KLF4, and SMO mutations are associated with classification and grading.15,16,18,19,52 AKT1 and SMO mutations characterize meningothelial meningiomas.45 KLF4 and TRAF7 alterations are linked to secretory meningiomas (Fig 9).53 Foramen magnum meningiomas (4/7, 57%) often have AKT1 mutations, making them suitable for targeted therapy.16 SMO-mutated olfactory groove meningiomas showed higher recurrence rates and larger tumor volumes in the anterior skull base than AKT1-mutated and wild-type meningiomas.17,40 Non-NF2-mutant meningiomas commonly present as benign, chromosomally stable, medial skull base tumors, contrasting with NF2-mutant tumors, which tend to be atypical, genomically unstable, and localized to the convexities.15,19 PIK3CA-mutant meningiomas showed limited chromosomal instability. Progesterone and cyproterone antiandrogen therapy showed higher PIK3CA mutation rates in skull base meningiomas.54,55 Meningiomas with POLR2A mutations are benign, exhibit distinct meningothelial histology, and tend to originate from the tuberculum sellae.51 Hedgehog tumors are typically midline, while non-NF2 tumors occur at the anterior skull base. KLF4-mutant meningiomas display more peritumoral edema. SMARCB1 meningiomas have a higher Ki-67 index.56 Radiation-induced meningiomas are often aggressive. These tumors usually lack NF2 mutations, and chromosome 1p loss plays an important role, followed by changes in 9p, 19q, and 22q locations.57
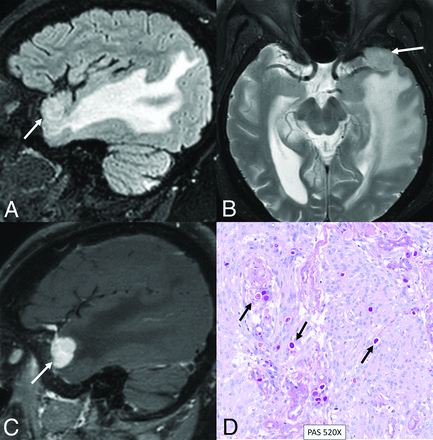
Secretory meningioma with a TRAF7 mutation in a 71-year-old woman. Sagittal FLAIR (A), axial T2-weighted (B), and sagittal contrast-enhanced (C) images reveal a small meningioma along the left greater wing of the sphenoid bone (arrows). Extensive parenchymal edema is noted in the right temporal lobe, disproportionate to the size of the meningioma. Histopathology revealed a secretory histologic subtype, with clusters of eosinophilic globules highlighted by pseudopsammoma bodies (D, arrows) with low mitotic activity (<1 mitosis in 10 high-power fields) and no atypical features, supporting a CNS WHO grade 1 designation. Next-generation sequencing studies demonstrated a pathogenic variant in TRAF7 (c.1136-1G>A).
H3K27 trimethylation inhibits tumorigenesis by regulating DNA repair and gene silencing.58 Loss of H3K27me3 trimethylation expression is rare (<5%).59,60 H3K27me3 loss is prevalent in grade 3 (37%) compared with grade 2 (20%) meningiomas, correlating with rapid progression and poor prognosis.35,61 Global methylation profiling predicts recurrence risk independent of histopathologic grade, resection extent, and copy-number alterations.59 Marastoni and Barresi62 proposed 3 classes to supplement WHO grading for prognostication. The first group lacks NF2 alterations and chromosomal instability, with mutations in AKT1, TRAF7, or KLF4 showing the best prognosis and response to cytotoxic drugs. The second group with intermediate prognosis has NF2 alterations and mild chromosomal instability. The third group with poor outcomes exhibits NF2 alterations, high chromosomal instability, and resistance to cytotoxic treatment, possibly with pTERT mutations and CDKN2A/B deletion.62 Low-grade meningiomas usually have isolated monosomy 22 or a balanced genome. In contrast, high-grade atypical and anaplastic meningiomas often have additional partial-arm chromosomal gains and losses, including loss of 1p, 6q, and 14q. Loss of 1p has been linked to higher rates of tumor recurrence and progression.27,63 The histologic subtypes of SMs are generally similar to cranial meningiomas. Genetic factors include NF2, SMARCB1, and TRAF7 gene alterations. NF2 homozygous deletion occurs in 80% of nonfamilial meningiomas and 100% of patients with NF2.20 NF2-mutant tumors are noted in the thoracic spine, with female predominance. AKT1-mutant tumors, mainly meningothelial, are more common in the cervical spine. SMARCE1 mutation is associated with multiple SMs and a clear-cell subtype.64,65 The Table summarizes commonly identified germline and somatic mutations in meningiomas by WHO grade, location, and clinical significance.
Imaging
On CT, meningiomas are sharply circumscribed homogeneous, iso- to hyperdense dural-based masses with homogeneous contrast enhancement, often with calcification and adjacent hyperostosis or osteolysis. Hyperostosis (25%–49%) is common with convexity (Fig 5) and sphenoid wing meningiomas.66 MRI is the preferred imaging method, providing essential features like tumor size, location, invasion, and recurrence, potentially eliminating the need for biopsy.7,67 Meningiomas typically are isointense to gray matter, with a contrast-enhancing dural tail sign often seen in reactive fibrovascular tissue, not necessarily indicating dural involvement. Vasogenic edema may be seen both with and without brain invasion. Prominent peritumoral edema is seen in secretory (Fig 9), angiomatous/microcystic, lymphoplasmacytic-rich, and high-grade meningiomas.56,68 Perfusion imaging generally reveals high relative CBF and relative CBV using the dynamic susceptibility contrast technique.69 On MRS, high alanine and low NAA levels are seen.70 The primary imaging differential includes primary brain tumors, inflammation, infections, and metastasis.70,71 A large study (1000 cases) found that only 2% of resected dural masses were nonmeningiomas.72 In a series by Nagai Yamaki et al,73 approximately 7.2% (25/348) of cases were meningioma mimics, including hemangiopericytoma/solitary fibrous tumor (48%), lymphoma (12%), and schwannoma. The authors highlighted 5 imaging red flags that can alert radiologists to consider meningioma mimics: 1) bone erosion (22.2%), 2) the dural displacement sign (36%), 3) marked T2 hypointensity (32%), 4) marked T2 hyperintensity (12%), and 5) absence of a dural tail (48%).73
Quantitative and qualitative MRI features can offer insights into tumor grades and clinical outcomes.74,75 A systematic review of 35 studies by Spille et al67 noted that irregular tumor shape, non-skull base location, heterogeneous enhancement, and tumor-brain interface disruptions were associated with grade 2 and 3 histology. Tumor and edema size usually correlates with recurrence, while heterogeneous contrast enhancement, cyst formation, T2-weighted intensity, and tumor capsule enhancement lack predictive value. A blurry brain/tumor surface with disruption of the peritumoral CSF cleft is supportive, however not definitive, of brain invasion (Fig 6).76 High-grade tumors show necrosis, hemorrhage, heterogeneity, nonspherical shape, and larger volumes. Radiomic (quantitative) and semantic (qualitative) classifiers demonstrated significant grade predictability (Area under curve semantic = 0.76 and Area under curve radiomic = 0.78).74 Similarly, clinical and radiologic features, such as symptoms, brain edema, shorter doubling time, and older age, predict high-grade meningiomas.77 Preoperative ADC values differentiate low-grade and high-grade meningiomas. In a meta-analysis of 25 studies with 1552 meningiomas (1102 low-grades, 450 high-grades), high-grade tumors had lower ADC values (0.79 versus 0.92). The ADC threshold achieved 69% sensitivity, 82% specificity, and an AUC of 0.84 for grade differentiation.78 Tumor volume is the primary predictor of higher-grade meningioma. Tumor necrosis and location along the falx or convexity may also independently predict higher-grade meningiomas.79 A combination of MR DTI parameters (Apparent diffusion coefficient minimum, fractional anisotropy, axial diffusivity, and radial diffusivity) accurately differentiates high-grade from low-grade meningiomas with 96.2% accuracy.80 On MRS, higher-grade tumors have high lipid and lactate peaks. However, they do not reliably differentiate typical and atypical meningiomas.81
FDG-PET/CT predicts recurrence. Lesions with minimal tracer uptake suggest favorable surgical outcomes, while hypermetabolism indicates atypical or recurrent meningiomas.82 Galium 68 (68Ga) DOTATATE PET/CT can help differentiate meningioma mimics, detect recurrence, plan radiation therapy, and monitor posttreatment effects (Figs 8 and 10).83⇓⇓–86 Sommerauer et al87 found a strong correlation between the maximum standard uptake value (SUVmax) and the tumor growth rate for grade 1 and 2 meningiomas. In contrast, grade 3 meningiomas showed lower SUVmax without a correlation with the tumor growth rate. Afshar-Oromieh et al88 observed 68Ga-DOTATOC uptake in all meningiomas (190), missing 10%, mainly along petroclival and falx cerebri. 68Ga DOTATATE PET/CT shows higher sensitivity (98.5% versus 53.7%) and specificity (86.7% versus 93.3%) for osseous involvement compared with MRI with transosseous meningiomas having larger volume (12.8 versus 3.3 mL; P < .001) and being more avid (SUVmax, 14.2 versus 7.6; P = .011).89
Rapid multifocal recurrence of CDKN2A/B-deleted CNS WHO grade 3 meningioma along the left sphenoid wing. The meningioma was initially classified as grade 2 (atypical) on the basis of histopathologic features; however, it was upgraded to grade 3 on the basis of detection of a homozygous deletion of CDKN2A/B on next-generation sequencing. Contrast-enhanced axial MRI depicts the left sphenoid wing meningioma (A, arrow) with intraosseous extension (asterisk). Rapid multifocal recurrence is noted on PET (DOTATATE) MRI (B–D, arrows) within a few months postresection.
Somatostatin receptor PET for residual tumor assessment surpasses intraoperative estimation via Simpson grading or MRI. In a post hoc analysis by Teske et al90 involving 46 patients with 49 grade 1 meningiomas, progression occurred in 14% of patients. 68Ga DOTATATE-positive PET (SUVmax > 2.3) was linked with progression (P = .015) and poor progression-free survival (P = .029), whereas MRI was not. All 20 patients with negative findings on PET remained recurrence-free. 68Ga-DOTATATE PET/MRI shows promise for planning and assessing focal radiation treatment for atypical and anaplastic meningiomas. A significant post-radiation therapy decrease in DOTATATE avidity (somatostatin receptor 2 expression) correlates with progression-free survival, highlighting its potential in evaluating radiation therapy response.84 Incorporating 68Ga DOTATATE PET into future trials could aid the clinician’s decision-making and enhance patient outcomes.85 The Online Supplemental Data detail 15 meningioma subtypes, covering histology, molecular characteristics, key imaging features, and clinical significance.
Prognosis and Treatment Strategies for Meningioma
Integrating histologic grading with genetic and epigenetic profiles provides a more accurate prognostic stratification, but it is not widely used in clinical practice.91 The extent of resection is a significant clinical predictor of recurrence and overall survival. The location, invasion, attachment to critical intracranial structures, and availability of expert neurosurgical services influence resection.92 Preoperative embolization of meningiomas reduces major surgical complications and improves follow-up.93 Advances in radiation therapy and image guidance allowed the safer delivery of higher doses without compromising treatment tolerance to unacceptable levels.91 High-grade meningiomas receiving adjuvant radiation therapy showed a higher overall recurrence rate than the stereotactic radiosurgery group (38% versus 25%, P = .01).94 Stereotactic radiosurgery effectively controls cerebellopontine angle meningiomas with minimal complications. Gendreau et al95 (meta-analysis of 6 studies, 406 patients) found 95.6% tumor control with low cranial nerve deficits. In SMs, complete surgical resection is the preferred treatment with low recurrence (1.3%−6.4%). Radiation therapy is used after subtotal resection and for grade 2 and 3 SMs.11,12 Patients with SMs have better 10-year survival than those with their benign (95.6% versus 83.2%) and malignant (73.4% versus 55.7%) cerebral counterparts.1
Meningioma recurrence has limited treatment options, with increasing neurologic worsening in patients undergoing first, second, and third surgeries. Repeat surgery should be considered when assessing the benefit-to-risk ratio.96 Despite advances in genomic and DNA methylation classification, treatment progress is slower.23 Somatostatin analogs, despite initial promise in recurrent and unresectable cases, in subsequent trials did not corroborate the benefits.97 In Phase II trials, peptide receptor radionuclide therapy (with 90Y- and 177Lu-DOTATOC) has demonstrated disease stabilization in progressive meningiomas.98 Systemic treatments, including antiangiogenic treatments and mammalian target of rapamycin (mTOR) inhibitors, have limited utility and are used for recurrent or progressive meningiomas.99 NF2 inactivation and mTOR overexpression focused on mTOR inhibitors (everolimus, combined with octreotide)100 or bevacizumab.101 PRRT has extended the 6-month PFS for grades 1 (89.7%) and 2 (57.1%) meningiomas.102 Limited treatment options and variable success rates have prompted several ongoing trials. These trials evaluate the possible therapeutic effects of immunotherapy, small molecule inhibitors, radionuclide therapy, and electrical field therapy for recurrent meningiomas (Online Supplemental Data). Management should be tailored individually, considering genetic changes that may favor systemic therapies. New WHO CNS5 grading and classification systems potentially influence management and outcomes like recurrence rates, albeit limited by the cost and availability of technologies such as DNA methylation and next-generation sequencing.
CONCLUSIONS
Most meningioma molecular biomarkers need further evaluation in prospective clinical trials. Their inclusion in the meningioma diagnosis and management may guide future targeted therapies. Adding CDKN2A/B and pTERT mutations in CNS5 classification is a step forward, particularly for grade 3 meningiomas. However, imaging biomarkers, including functional imaging like PET/CT, are still in their infancy in predicting tumor grades and histopathologic subtypes. CNS5 classification with multimodality imaging may improve the prediction of the clinical course of meningiomas. This has yet to yield meaningful therapeutic advancements. Ongoing effort aims to translate molecular knowledge into clinical management.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- 95.↵
- 96.↵
- 97.↵
- 98.↵
- 99.↵
- 100.↵
- 101.↵
- 102.↵
- Received April 29, 2024.
- Accepted after revision June 1, 2024.
- © 2025 by American Journal of Neuroradiology