Abstract
BACKGROUND AND PURPOSE: The human brain displays structural and functional disparities between its hemispheres, with such asymmetry extending to the frontal aslant tract. This plays a role in a variety of cognitive functions, including speech production, language processing, and executive functions. However, the factors influencing the laterality of the frontal aslant tract remain incompletely understood. Handedness is hypothesized to impact frontal aslant tract laterality, given its involvement in both language and motor control. In this study, we aimed to investigate the relationship between handedness and frontal aslant tract lateralization, providing insight into this aspect of brain organization.
MATERIALS AND METHODS: The Automated Tractography Pipeline was used to generate the frontal aslant tract for both right and left hemispheres in a cohort of 720 subjects sourced from the publicly available Human Connectome Project in Aging database. Subsequently, macrostructural and microstructural parameters of the right and left frontal aslant tract were extracted for each individual in the study population. The Edinburgh Handedness Inventory scores were used for the classification of handedness, and a comparative analysis across various handedness groups was performed.
RESULTS: An age-related decline in both macrostructural parameters and microstructural integrity was noted within the studied population. The frontal aslant tract demonstrated a greater volume and larger diameter in male subjects compared with female participants. Additionally, a left-side laterality of the frontal aslant tract was observed within the general population. In the right-handed group, the volume (P < .001), length (P < .001), and diameter (P = .004) of the left frontal aslant tract were found to be higher than those of the right frontal aslant tract. Conversely, in the left-handed group, the volume (P = .040) and diameter (P = .032) of the left frontal aslant tract were lower than those of the right frontal aslant tract. Furthermore, in the right-handed group, the volume and diameter of the frontal aslant tract showed left-sided lateralization, while in the left-handed group, a right-sided lateralization was evident.
CONCLUSIONS: The laterality of the frontal aslant tract appears to differ with handedness. This finding highlights the complex interaction between brain lateralization and handedness, emphasizing the importance of considering handedness as a factor in evaluating brain structure and function.
ABBREVIATIONS:
- AD
- axial diffusivity
- EHI
- Edinburgh Handedness Inventory
- FA
- fractional anisotropy
- FAT
- frontal aslant tract
- FDR
- false discovery rate
- HCP
- Human Connectome Project
- HCP-A
- HCP Lifespan in Aging
- ICV
- intracranial volume
- LH
- left-handed
- LI
- lateralization index
- MD
- mean diffusivity
- RD
- radial diffusivity
- RH
- right-handed
SUMMARY
PREVIOUS LITERATURE:
Several studies demonstrated a left-side laterality of FAT and other language-related tracts, such as the arcuate fasciculus. Handedness is proposed to influence brain asymmetries, though findings on handedness-related asymmetry within brain structures have been contradictory. Studies have shown a leftward white matter asymmetry within the inferior frontal and precentral gyrus, which connect with FAT, in right-handed individuals, while no asymmetry was observed in left-handed individuals. Handedness-dependent laterality has also been observed in the dorsal component of the superior longitudinal fasciculus. However, the impact of handedness on FAT laterality, considering its roles in both language and motor control, remains unexplored.
KEY FINDINGS:
The laterality of the FAT appears to vary among different handedness groups, with left-handed individuals demonstrating dominance in both the volume and diameter of the tract toward the right hemisphere, while right-handed individuals exhibit dominance toward the left hemisphere.
KNOWLEDGE ADVANCEMENT:
Our study demonstrates that asymmetry in the FAT depends on individual handedness, enhancing our understanding of the mechanisms underlying brain lateralization. Recognizing this is crucial in research involving white matter tract structures and holds clinical implications, particularly in neurosurgical planning and intervention.
The frontal aslant tract (FAT) is a recently identified white matter bundle that connects the pars opercularis and pars triangularis of the inferior frontal gyrus to the supplementary motor area and presupplementary motor area.1 The FAT plays a role in a variety of cognitive functions, including speech production, language processing, and executive functions. Specifically, the left FAT is highly involved in language production, particularly in speech initiation and sequencing,2⇓-4 and the right FAT is associated with inhibitory control in executive function tasks.5,6
The human brain displays both structural and functional asymmetry across its hemispheres,7,8 a characteristic that also extends to the FAT. The laterality of the FAT refers to the degree to which it is more developed on one side of the brain compared with the other. Some studies have found that the FAT is more lateralized on the left side.1,9⇓-11 The underlying reasons for the laterality of the FAT remain incompletely understood. However, one hypothesis suggests that this asymmetry could be associated with the distinct functions of the hemispheres of the brain. The right hemisphere is commonly linked to visuospatial processing, whereas the left hemisphere is often associated with language processing.12
Handedness, the preference for using one hand over the other, has long interested scientists due to its potential implications for brain organization and function. Studies suggest a correlation between brain structural and functional asymmetries and handedness. More than 95% of right-handed (RH) individuals and approximately 76% of left-handed (LH) individuals exhibit left-hemisphere dominance for language function.13,14 This phenomenon prompts inquiries into whether and how handedness relates to the lateralization of neural structures involved in both language and motor control, such as the FAT.
Recent advancements in neuroimaging techniques, such as DTI-based streamline tractography, have contributed substantially to unraveling the complexity of brain structures and lateralization of the white matter tract.15 While most diffusion asymmetry investigations have predominantly concentrated on RH subjects,16 there is evidence supporting the hypothesis that LH individuals exhibit atypical structural asymmetries.17,18 However, the influence of handedness on FAT laterality remains relatively underexplored, highlighting the need for further investigation to clarify the exact nature of these relationships and enhance our understanding of the mechanisms underlying brain lateralization. Such insights could offer potential clinical applications in neurosurgical planning and intervention.
Motivated by the gaps in current understanding, we aimed to investigate the effect of handedness on FAT laterality. We hypothesized that handedness will modulate the asymmetry of the FAT, with a greater laterality toward the left hemisphere in RH individuals compared with LH individuals, analogous to the asymmetry observed in language functional activation. We leveraged the Human Connectome Project (HCP) Lifespan data to investigate the laterality of the FAT and conducted a comparative analysis among different handedness groups. Furthermore, we explored potential age-related and sex-related differences in this specific white matter tract.
MATERIALS AND METHODS
Subjects
We obtained neuroimaging data of 720 participants from the publicly available HCP in Aging (HCP-A) Lifespan 2.0 release (https://www.humanconnectome.org/study/hcp-lifespan-aging/article/lifespan-20-release-hcp-aging-hcp-development-data).19 The mean (SD) age of participants was 60.4 (15.7) years, ranging between 36 and 100 years, and 56.1% were women. HCP aging collect MRI data from multiple institutes including: Washington University, University of Minnesota, Massachusetts General Hospital, Harvard University, University of California Los Angeles, Oxford University.
Handedness
The Edinburgh Handedness Inventory (EHI) scores of each participant were obtained from the HCP behavioral database to evaluate handedness.20 The EHI is a questionnaire assessing the preferred hand for performing various tasks. The scores range from −100 to 100, in which negative values indicate left-handedness and positive values indicate right-handedness. These EHI scores were used to categorize handedness as follows: Scores below −40 indicated left-handedness, scores between −40 and 40 indicated ambidexterity, and scores exceeding +40 indicated right-handedness.21⇓-23
MR Imaging Acquisition
The diffusion MR imaging data in HCP-A Projects were obtained using a Magnetom Prisma 3T scanner (Siemens) equipped with a 32-channel Prisma head coil (Siemens).19 Spin-echo EPI sequences were acquired with the following parameters: TR/TE = 3230/89.20 ms, flip angle = 78°, 1.5-mm isotropic resolution, field of view = 210 × 180, matrix = 140 × 120, 92 slices, and a multiband acceleration factor of 4. A total of 185 diffusion-weighting directions were acquired, including 2 shells with b-values of 1500 and 3000 s/mm2, along with 28 images with b=0 s/mm2. The scanning time for the DTI acquisition was approximately 21 minutes.24
Tractography
The Automated Tractography Pipeline provided by DSI studio (http://dsi-studio.labsolver.org), which has demonstrated good levels of reproducibility in previous research,9 was used to generate the right and left FAT in each subject. In summary, a multishell diffusion scheme was used with b-values of 1500 and 3000 s/mm2. The number of diffusion sampling directions was 93 and 92, respectively. The in-plane resolution and section thickness were both 1.5 mm. The diffusion MR imaging data underwent preprocessing, including eddy current and motion correction, as well as phase distortion correction, and then were reconstructed in the Montreal Neurological Institute space using q-space diffeomorphic reconstruction25 to obtain the spin distribution function with a diffusion sampling length ratio of 1.25. Subsequently, seeds were placed in the HCP tractography atlas tract volume,26 and a deterministic fiber-tracking algorithm27 was used to generate streamlines, followed by topology-informed pruning with 48 iterations to eliminate false connections.28 Shape analysis was then conducted to derive shape metrics for tractography.9 Macrostructural properties of each tract, such as the number of streamlines, volume, length, and diameter as well as microstructural integrity parameters including fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD), were investigated. To assess the asymmetry of the tracts, we calculated a lateralization index (LI) for each parameter using the following formula: LI = (right – left) / (right + left) × 100.29 Consequently, positive LI values indicate rightward lateralization, while negative values indicate leftward lateralization.
Statistical Analyses
All statistical analyses were conducted using SPSS (Version 28; IBM). Partial correlation analysis was conducted to investigate the association between age and FAT parameters, while controlling for sex and intracranial volume (ICV). Brain-extracted restricted diffusion imaging was used to generate a brain mask, and ICV was computed using FSL software (Version 6.0.5; http://www.fmrib.ox.ac.uk/fsl) for all subjects. Univariate generalized linear models were used to compare macrostructural and microstructural parameters of the FAT between sexes, considering age and ICV as covariates. To compare the right and left FAT in the general population and each handedness group, we used paired t tests. To compare LIs between RH and LH subjects, we used univariate generalized linear models, controlling for sex, age, and ICV. False discovery rate (FDR) correction was applied to adjust the significance level in multiple comparisons. A P value < .05 was considered statistically significant.
RESULTS
Age-Related Change in FAT
After controlling for sex and ICV, we observed a negative correlation among the number of streamlines (left FAT: P = .004 and right FAT: P = .037), volume (left FAT: P < .001 and right FAT: P = .042), and diameter (left FAT: P < .001 and right FAT: P = .016) with age in the left and right FAT. Additionally, FA values demonstrated a decrease (left and right FAT: P < .001), while diffusivity values (MD, AD, and RD) exhibited an increase with aging (left and right FAT: P < .001) (Online Supplemental Data).
Sex-Related Difference in FAT
The cohort consisted of 404 (56.1%) women and 316 (43.9%) men. Table 1 illustrates that among male subjects, the left FAT demonstrated greater volume (P = .002) and a larger diameter (P = .003) compared with female subjects, while controlling for age and ICV. Similarly, the right FAT exhibited higher volume (P = .001) and a larger diameter (P = .004) in male subjects compared with female subjects. However, there were no significant differences in the microstructural integrity parameters between male and female subjects.
Comparing FAT parameters across sex groups, controlling for age and ICV
Comparing Right and Left FAT
Table 2 demonstrates the comparative analysis of the right and left FAT in all subjects. The mean left FAT volume was 16,614 (SD, 2992) mm3, with a mean length of 75.6 (SD, 3.7) mm. The right mean FAT volume was 16,286 (SD, 3039) mm3, with a diameter of 75.0 (SD, 3.6) mm. The volume (P = .003) and length (P < .001) of the left-side FAT were significantly higher than those of the right side. Furthermore, the FA values were significantly higher (P < .001), whereas the MD and RD values were significantly lower (P < .001) in the left FAT.
Macrostructural and microstructural differences between right- and left-sided FAT
Comparing FAT across Different Handedness Groups
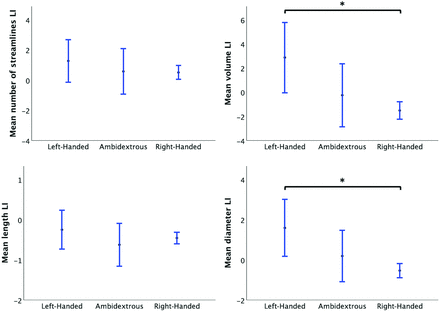
On the basis of EHI scores, 612 participants (57.7% women) were categorized as right-handed; 55 participants (43.6% women), as left-handed; and 53 participants (50.9% women), as ambidextrous. We compared the right and left FAT in each handedness group, while controlling for age, sex, and ICV. In the RH group, the left mean FAT volume (16,615 [SD, 2929] mm3), length (75.6 [SD, 3.7] mm), and diameter (16.7 [SD. 1.3] mm) were observed to be higher compared with the mean right FAT volume (16,153 [SD, 3006] mm3), length (74.9 [SD, 3.6] mm), and diameter (16.5 [SD, 1.4] mm). In contrast, in the LH group, the mean left FAT volume (16,331 [SD, 3654] mm3) and diameter (16.4 [SD, 1.8] mm) were lower compared with the right FAT volume (17,200 [SD, 2958] mm3) and diameter (16.9 [SD, 1.3] mm). These differences in the LH group did not remain statistically significant after applying the FDR correction for multiple comparisons. FA values were higher in the left FAT in both RH (P < .001) and LH (P < .001) groups compared with the right FAT. In contrast, MD and RD values of left FAT were lower in both RH (MD: P < .001, RD: <.001) and LH (MD: P < .006, RD: P < .001) groups compared with the right FAT (Table 3 and Fig 1). Subsequently, we compared the LI of different FAT parameters across each group. In the RH group, the mean LI of FAT volume and diameter was negative (lateralized to the left). While in the LH group, the mean LI of FAT volume and diameter was positive (lateralized to the right). LIs of volume (P = .001) and diameter (P < .001) were significantly different across handedness groups (Table 4 and Figs 2 and 3). Additionally, the EHI scores correlated negatively with the volume LI (P = .001) and diameter LI (P = .001), after controlling for age, sex, and ICV (Online Supplemental Data).
Comparing left and right FAT parameters in each handedness group. Significant differences (FDR-corrected P < .05), after controlling for age, sex, and ICV, are indicated with asterisks. The error bars represent 95% CIs.
Comparing the LI of macrostructural parameters across handedness groups. Significant differences (FDR-corrected P < .05), after controlling for age, sex, and ICV, are indicated with asterisks. The error bars represent 95% CIs.
A, The right and left FAT in a sample RH subject (EHI: +100) demonstrating left-side dominance in FAT volume. B, Right and left FAT in a sample LH subject (EHI: −70) demonstrating right-side dominance in FAT volume. L indicates left; R, right.
Comparison of FAT parameters across handedness groups
Comparison LI across handedness groups
DISCUSSION
In the present study, we investigated the variability of the FAT across diverse age and sex groups and performed a comparative analysis of the FAT laterality in relation to handedness in the cohort of 720 participants of the HCP Lifespan study. The FAT showed an age-related reduction in both the macrostructural and microstructural properties. Notably, the macrostructural parameters of the FAT were found to be more pronounced in the male group, while no significant sex-related differences were observed in the microstructural parameters. In general, the FAT was dominant in the left hemisphere; however, our results suggest that this laterality is dependent on handedness. Specifically, within the LH group, we found a higher volume and diameter of the right FAT in comparison with the left FAT.
Age-related alterations in white matter tracts were previously reported in quantitative DTI tractography studies. A nonlinear reduction in FA values and a concurrent elevation in diffusivity values (MD, AD, and RD) were demonstrated in major projection, association, and commissural white matter pathways.30⇓–32 Notably, the prefrontal white matter was found to be most vulnerable to the aging process.32 Our study aligns with these findings, because we found a similar pattern of age-related changes in the FAT in both cerebral hemispheres. This loss of macrostructural and microstructural organization with normal aging may be related to white matter change, including tract atrophy, a decline in myelinated fibers, and an increase in white matter hyperintensity lesions.33⇓–35
The observed difference in FAT macrostructural parameters between male and female subjects adds to our understanding of brain sexual dimorphism. We observed a larger FAT volume and diameter in men. Greater within-hemispheric structural connectivity and a larger number of streamlines were demonstrated in all supratentorial regions of male subjects.36,37 Eikenes et al38 investigated the sex differences in white matter tracts in the ICV‐matched group and found a greater tract volume of the uncinate fasciculus in men compared with women. This discrepancy suggests the influence of biologic and hormonal factors associated with sex on shaping the development and maintenance of neural pathways. Testosterone, which is typically more abundant in males, has been implicated in brain development and connectivity,39 which could potentially contribute to the observed larger FAT in men. Further research is needed to determine the exact cause of the sex difference in FAT volume and diameters. In our study, no sex-related difference was detected in the microstructural integrity of the FAT. This result aligns with the findings of Kitamura et al,40 who also did not identify significant differences in DTI parameters across sex groups for various other white matter tracts, including the cingulate fasciculus, superior longitudinal fasciculus, inferior longitudinal fasciculus, and the inferior occipitofrontal fasciculus. However, they did observe sex differences in the FA values for the right uncinate fasciculus. Additionally, in a separate study, Szeszko et al41 demonstrated higher FA values in the left frontal lobe white matter of women compared with men, though they did not specifically assess the different frontal lobe white matter tracts as distinct entities. These combined findings enhance our understanding of sex-related neurodevelopmental disparities in the macrostructural parameters of white matter tracts, highlighting the importance of incorporating sex as a crucial variable in research focused on brain structure and function.
Several studies demonstrated the left-side laterality of the FAT in terms of macrostructural parameters.1,9⇓–11 The same lateralization has also been observed for other language-related tracts, including the arcuate fasciculus.9,42 In line with previous work, we found a larger volume and length of the left FAT as well as a higher FA value and lower MD on the left side. Furthermore, we now demonstrate that this asymmetry is contingent on individual handedness, in which the volume and diameter of the FAT is lateralized to the right side in LH subjects. In RH subjects, the left FAT exhibited higher volume, diameter, and FA values compared with the right FAT, while MD and RD values were lower on the left side. These observations suggest structural asymmetries favoring the left hemisphere in RH subjects, potentially indicative of increased axonal density or myelination with greater structural integrity and organization of the left FAT. These findings align with existing literature indicating structural dominance of language-related regions and pathways in the left hemisphere of RH individuals.43 In the LH group, the right FAT had a higher volume and diameter compared with left FAT. However, similar to the findings in the RH group, the right FAT demonstrated lower FA values and higher MD and RD values.
These results suggest a trend wherein the right FAT tends to be larger but exhibits lower structural integrity compared with the left side in the LH group. However, confirmation of this trend would benefit from a study with a larger sample size of LH subjects. The more pronounced statistical differences seen when comparing the volume and diameter of the left FAT with those of the right FAT within the RH group compared with the LH group may be due to differences in the sample size. Additionally, the notable reductions in AD observed in the right FAT among RH subjects, not evident in the LH group, could also be linked to sample size disparities between the groups. The current findings warrant validation through larger sample sizes in future studies. Previous studies using voxel-based morphometry techniques have had controversial findings on handedness-related asymmetry within brain structures. Several investigations have reported an absence of asymmetry in both gray and white matter associated with handedness.44⇓–46 However, Hervé et al18 identified leftward white matter asymmetry within the inferior frontal gyrus and precentral gyrus, which connect with the FAT among RH subjects, whereas neither asymmetry nor rightward asymmetry was observed in the LH group. Similar handedness-dependent laterality that we found for the FAT volume was previously reported for the dorsal component of the superior longitudinal fasciculus, which connects the superior parietal lobule and the superior frontal gyrus. The volume of the superior longitudinal fasciculus was lateralized to the left in RH subjects and to the right in LH subjects.47
The relationship between human handedness and the structure of white matter tracts is not yet fully understood. One hypothesis proposes that tract lateralization is contingent on its functional roles. Although several studies have suggested asymmetry in the FAT function, with the left FAT being implicated in the speech and language domain and the right FAT being associated with executive and inhibitory control,48 recent research indicates that both the left and right FAT play a role in both language and executive functions.49 For instance, there is evidence of the right FAT being involved in language function; the integrity of the right FAT has been linked to speech fluency in children with autism and the severity of stuttering in adults.50,51 Most interesting, intraoperative electrical stimulation of the right FAT induced paraphasia and speech arrest.52
Understanding how handedness interacts with FAT laterality not only contributes to our knowledge of the mechanisms underlying brain lateralization but also holds clinical implications, particularly in neurosurgical planning and intervention. Recognizing the asymmetry between RH and LH subjects during presurgical planning for procedures involving the frontal lobe, such as tumor resections or epilepsy surgeries, enables surgeons to customize approaches on the basis of the laterality of the FAT relative to the patient’s handedness. This customization helps minimize the risk of intraoperative unintended damage and preserves functional integrity, ultimately improving surgical outcomes and patient care.
Furthermore, understanding the baseline anatomic properties such as hemispheric dominance could be important in cases of potential damage, analogous to task functional dominance. While in individual subjects, FAT laterality correlates with handedness (much like language activation), there may be cases in which bilaterally equal representation of the FAT may confer greater resistance to tract-induced functional deficits. Future studies can thus explore the relationship between FAT laterality and outcome in cases of tract injury.
On the basis of these findings, further task-dependent fMRI studies involving RH and LH subjects are needed to explain the mechanism underlying the different laterality of the FAT observed in this study. This study provides us with insight into how the FAT varies across different handedness profiles and presents an opportunity for further exploration into the mechanisms regulating the interplay between neural architecture and handedness.
This study has several limitations. The number of LH participants in the sample was smaller than the number of RH participants. However, this study included a greater number of LH participants compared with prior studies focused on this group.16 Additionally, the deterministic fiber-tracking technique used in this study is more prone to generating false-negative results. It is possible that minor branches may have gone undetected.17 Furthermore, the tract recognition algorithm in DSI Studio used a tractography atlas as the sole reference, without using cortical regions as ROIs. The developer’s rationale was to avoid concerns associated with cortical parcellations. Further investigation is required to determine whether this approach yields superior or inferior performance.
Another limitation is the absence of functional imaging in this study to enable a comparison of the functional asymmetry of the FAT between the 2 groups of handedness. Finally, while the results show statistical significance, caution must be exercised in individual analysis due to the range of overlap among distinct groups of comparison.
CONCLUSIONS
The laterality of the FAT appears to vary between different handedness groups, with LH individuals demonstrating dominance in both the volume and diameter of the tract toward the right side. This comparative analysis highlights the significance of considering handedness when studying the structure of white matter tracts. Future studies that combine structural and functional imaging are essential to enhance our understanding of the relationship between the laterality of the FAT and handedness.
Acknowledgments
The HCP-A Lifespan 2.0 Release data used in this report came from https://brain.labsolver.org/hcp_a.html.
Footnotes
Diffusion MR imaging data used in the preparation of this article were obtained from the Lifespan Human Connectome Project in Aging (HCP-A). The HCP-A Project was supported by the National Institute on Aging of the National Institutes of Health under award No. U01AG052564 and by funds provided by the McDonnell Center for Systems Neuroscience at Washington University in St. Louis.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received December 22, 2023.
- Accepted after revision February 28, 2024.
- © 2024 by American Journal of Neuroradiology