Abstract
BACKGROUND AND PURPOSE: Hemorrhagic transformation can occur as a complication of endovascular treatment for acute ischemic stroke. This study aimed to determine whether ischemia depth as measured by admission CTP metrics can predict the development of hemorrhagic transformation at 24 hours.
MATERIALS AND METHODS: Patients with baseline CTP and 24-hour follow-up imaging from the ESCAPE-NA1 trial were included. RAPID software was used to generate CTP volume maps for relative CBF, CBV, and time-to-maximum at different thresholds. Hemorrhage on 24-hour imaging was classified according to the Heidelberg system, and volumes were calculated. Univariable and multivariable regression analyses assessed the association between CTP lesion volumes and hemorrhage/hemorrhage subtypes.
RESULTS: Among 408 patients with baseline CTP, 142 (35%) had hemorrhagic transformation at 24-hour follow-up, with 89 (63%) classified as hemorrhagic infarction (HI1/HI2), and 53 (37%), as parenchymal hematoma (PH1/PH2). Patients with HI or PH had larger volumes of low relative CBF and CBV at each threshold compared with those without hemorrhage. After we adjustied for baseline and treatment variables, only increased relative CBF <30% lesion volume was associated with any hemorrhage (adjusted OR, 1.14; 95% CI, 1.02–1.27 per 10 mL), as well as parenchymal hematoma (adjusted OR, 1.23; 95% CI, 1.06–1.43 per 10 mL). No significant associations were observed for hemorrhagic infarction.
CONCLUSIONS: Larger “core” volumes of relative CBF <30% were associated with an increased risk of PH following endovascular treatment. This particular metric, in conjunction with other clinical and imaging variables, may, therefore, help estimate the risk of post-endovascular treatment hemorrhagic complications.
ABBREVIATIONS:
- AUC
- area under the curve
- eTICI
- expanded TICI
- EVT
- endovascular treatment
- HI
- hemorrhagic infarction
- IQR
- interquartile range
- PH
- parenchymal hematoma
- rCBF
- relative CBF
- sICH
- symptomatic intracerebral hemorrhage
- Tmax
- time-to-maximum
Hemorrhagic transformation of ischemic stroke is common and part of the natural history. A large percentage of hemorrhagic transformations are asymptomatic, inconsequential to prognosis.1,2 They are associated with reperfusion therapy, thrombolysis, and endovascular treatment (EVT) and appear within 24 hours when these therapies are performed.3 Radiologically, hemorrhagic transformation can range in severity from small petechial hemorrhage without noticeable mass effect to larger, space-occupying parenchymal hematoma (PH).4 The presence of PH is unequivocally associated with worse outcomes and is symptomatic.5⇓-7 PH occurs more commonly when there is a lack of early reperfusion.
Larger volumes of increasing ischemia depth as measured by the CTP parameters prolonged mean transit time, prolonged time-to-maximum (Tmax), and relative CBF (rCBF) may indicate impaired collateral circulation and an increased risk of hemorrhagic transformation.8⇓-10 However, the current literature presents conflicting data on optimal CTP parameter thresholds for the prediction of hemorrhagic transformation, most studies being based on retrospective or observational analyses of small cohorts.11
The aim of this study was to investigate the association between CTP-derived lesion volumes and the occurrence of hemorrhagic infarction (HI) or PH at 24 hours post-EVT using data from a randomized controlled trial.
MATERIALS AND METHODS
Patient Sample
Data are from the Safety and Efficacy of Nerinetide in Subjects Undergoing Endovascular Thrombectomy for Stroke (ESCAPE-NA1) trial, registered under clinicaltrials.gov with the identifier NCT02930018.12 ESCAPE-NA1 was a double-blind, multicenter randomized controlled trial that aimed to evaluate the efficacy of nerinetide in patients with acute ischemic stroke who underwent EVT.
Patients were randomly assigned to receive either IV nerinetide or a placebo in addition to best medical management, including IV alteplase if deemed appropriate. The inclusion criteria for the parent trial were as follows: 1) 18 years of age or older with a large-vessel occlusion (intracranial ICA, MCA M1 or all M2 branches), 2) baseline NIHSS score of more than five, 3) time from the last seen well to randomization within 12 hours, 4) functional independence before the stroke, 5) moderate-to-good collateral circulation, and 6) ASPECTS of >4. All patients underwent NCCT and single-phase or multiphase CTA at baseline.
For the current study, only patients who had baseline CTP imaging, performed as part of clinical routine at each respective site but not mandated by the trial, were included in the analysis. The participating sites obtained appropriate ethics and local regulatory approval, and informed consent was obtained from the participants, legally authorized representatives, or investigators, following the requirements of national laws or regulations, including 2-physician consent when necessary.
Imaging Analysis
All imaging data were evaluated by a central imaging core lab, which was blinded to treatment allocation and clinical outcomes. The baseline NCCT scan was used to assess the ASPECTS. Collateral circulation was evaluated on CTA and categorized as poor, moderate, or good. The location of the occlusion was reported as the terminal ICA, M1 segment of the MCA, or M2 segment of the MCA.
Perfusion source images were processed using RApid processing of PerfusIon and Diffusion (RAPID software, Version 5.2.2; iSchemaView) to generate rCBF, CBV, and Tmax volumes. Each volume was provided at specific standard thresholds. The output DICOM files were converted to NIfTI format by using dcm2niix (http://www.github.com/rordenlab/dcm2niix) and underwent automated segmentation using color-based thresholding in Python (Version 3.10). The segmentation volumes were extracted using 3D Slicer, Version 5.0.2 (http://www.slicer.org). These additional processing steps were performed to provide more detail regarding the affected brain regions at each threshold, allowing more precise segmentation/volume calculation. Key Python functions necessary for reproduction of feature extraction and processing are detailed on Github (https://github.com/naterex23/RAPID_Perfusion_Processing), and an additional Python source code is available on reasonable request.
Secondary CTP-based metrics, including the hypoperfusion intensity ratio, mismatch, and mismatch ratio, were calculated. The hypoperfusion intensity ratio represents the volume of Tmax >10 seconds divided by the volume of Tmax >6 seconds. The mismatch is calculated as the volume of Tmax >6 seconds minus the volume of rCBF <30%, and the mismatch ratio is the volume of Tmax >6 seconds divided by the volume of rCBF <30%.
The evaluation of the expanded TICI (eTICI) was performed on the final intracranial DSA run. The presence and volumes of any hemorrhagic transformation were determined as described by Ospel et al.7 Briefly, hemorrhagic transformation was assessed through visual inspection of the 24-hour follow-up imaging by an interventional neuroradiologist (M.G., with 24 years of experience) and a general radiologist (J.M.O., with 4 years of experience). Discrepancies were resolved by consensus. Hemorrhagic transformation was classified into 4 subtypes: HI types 1 and 2 and PH types 1 and 2, according to the Heidelberg criteria.4 Due to their infrequency, remote parenchymal hematomas (n = 3) were included in the PH groups. For this analysis, HI-1 and HI-2 were combined, as were PH-1 and PH-2. Symptomatic intracerebral hemorrhage (sICH) was defined as any hemorrhage associated with clinical evidence of neurologic worsening, with the hemorrhage considered the main cause of the decline.13
Outcome Measures
The primary outcome was the presence of any intracranial hemorrhage at 24 hours. Secondary outcomes included the presence of HI1 or HI2 and the presence of PH1 or PH2. sICH was analyzed as a safety outcome.
Statistical Analysis
Baseline characteristics and treatment factors of the participants were described using descriptive statistics as appropriate to the type and distribution of the data. Comparisons were made between participants with and without any hemorrhage at follow-up imaging.
Unadjusted comparisons of CTP-derived lesion volumes at baseline between patients with and without outcomes of interest were made using nonparametric tests. Adjusted effect size estimates for associations of CTP-derived lesion volumes and outcomes were obtained using multivariable logistic regression. The multivariable regression models were adjusted for age, sex, baseline glucose level, NIHSS, ASPECTS, collateral score, alteplase administration, successful reperfusion (eTICI 2c/3), time to reperfusion, and procedural complications. Separate models were constructed for the RAPID-generated CTP parameters rCBF <30%, Tmax > 6 seconds, and CBV <38%. These specific rCBF and Tmax thresholds were chosen because they represent the RAPID standard output for core and penumbra, respectively, while CBV <38% was chosen as a midrange indicator of ischemia depth.
Statistical analyses were performed using STATA 17 software (Stata Corp), and a level of P < .05 was considered statistically significant. No imputation was performed for minimal missing data. Finally, because this was an exploratory subgroup analysis, no formal power analysis was performed, and all results are considered exploratory.
RESULTS
Patient Characteristics
Presence of Any Hemorrhage.
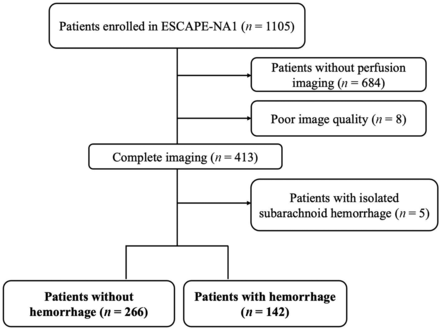
Among the 1105 patients enrolled in the trial, baseline CTP source imaging was available for 421. Eight patients were excluded from the CTP analysis due to low scan quality, and 5 patients had isolated subarachnoid bleeds, resulting in a total of 408 patients included in the analysis (Figure). The median age of the patients was 70.1 years (interquartile range [IQR], 60.3–79.8 years), with 50% of them being women. Hemorrhage on follow-up imaging, observed in 142 patients (35%), was determined by segmented volumes from either CT (72 patients, 51%) or MR imaging (70 patients, 49%) at 24 hours. The Online Supplemental Data provide an overview of baseline clinical, imaging, treatment, and outcome variables for patients with and without hemorrhage, further stratified by the type of bleed (HI1/HI2 or PH1/PH2).
Flow chart of inclusion.
Patients with evidence of any intracranial hemorrhage on follow-up imaging (n = 142) had higher admission blood glucose levels (median, 7.1 mg/dL [IQR, 6.2–9.0 mmol/L] versus 6.6 mmol/L [IQR, 5.8–7.6 mmol/L]; respectively, P < .001), higher baseline NIHSS scores (median, 18 [IQR, 15–21] versus 17 [IQR, 12–20]; P = .007), lower baseline ASPECTS (median, 8 [IQR, 6–8] versus 8 [IQR, 7–9]; P < .001), and worse collateralization (15 of 141 participants [10.6%] versus 44 of 261 participants [16.7%] with good collateral vessels; P = .022). Regarding treatment, patients with evidence of any intracranial hemorrhage had longer onset-to-reperfusion times (median, 332.5 minutes [IQR, 214–550.5 minutes] versus 210 minutes [IQR, 158.5–297 minutes]; P < .001) and achieved successful recanalization less frequently (52 of 142 participants [36.6%] versus 131 of 263 participants [49.8%]; P = .012). There were no differences in alteplase administration observed (Online Supplemental Data).
Hemorrhage Subtypes.
Within this cohort, most observed hemorrhages were classified as either HI1 (52 of 142, 36.6%) or HI2 (37, 26.0%). PH1 and PH2 accounted for 23.2% (33 of 142) and 14.1% (20 of 142), respectively. At 24 hours, sICH was present in 14 of 142 (9.9%) patients (Online Supplemental Data). When stratifying according to bleeding type, the significant differences in baseline characteristics between cohorts with any hemorrhage versus none and patients with HI1/HI2 versus none remained, except for the rate of successful reperfusion, which was no longer significant in the latter (35 of 89 [39.3% versus 49.8%], respectively; P = .110) (Online Supplemental Data).
For patients with PH1/PH2, the baseline ASPECTS was lower (7.5 [IQR, 6–8] versus 8 [IQR, 7–9]; P = .041). In terms of procedural characteristics, patients with PH1/PH2 had lower rates of successful reperfusion (17 [32.1%] versus 131 [49.8%]; P = .023) and longer onset-to-reperfusion times (median, 394 minutes [IQR, 261–578 minutes] versus 311 minutes [IQR, 209–540 minutes]; P < . 001) compared with those without any hemorrhage. Overall, few differences were observed between patients with and without sICH, with the former group generally having higher baseline systolic blood pressure (median, 157 mm Hg [IQR, 140–190 mm Hg] versus 144 mm Hg [IQR, 129–161 mm Hg]; P = . 027) (Online Supplemental Data).
Perfusion-Based Characteristics
Presence of Any Hemorrhage.
Significant differences in volume were observed at the rCBF <30% and CBV <38% thresholds between patients with any hemorrhage and those without at follow-up (Online Supplemental Data). In both cases, the hemorrhage group exhibited larger deficit volumes (rCBF <30%; median, 17.9 mL [IQR, 6.4–43.9 mL] versus rCBF <30%: 6.1 mL [IQR, 0.0–22.5 mL]; P < . 001, and CBV <38%: median, 17.4 mL [IQR, 5.1–45.4 mL] versus CBV <38%: 6.9 mL [IQR, 0.0–32.7 mL]; P < . 001, respectively). Although Tmax > 6-second volumes were numerically larger in the hemorrhage group, the difference was not significant (Online Supplemental Data).
Univariable regression analyses revealed significant associations between both rCBF <30% (OR, 1.17; 95% CI, 1.09–1.26; P < .001, area under the curve [AUC], 0.64) and CBV <38% (OR, 1.09; 95% CI, 1.03–1.16; P = .003, AUC, 0.61) thresholds and the presence of any hemorrhage at follow-up (Table 1). After adjusting for predefined variables, only the associations between rCBF <30% and hemorrhage at follow-up remained. Once again, no significant associations were found for Tmax > 6 seconds (Table 2).
Unadjusted associations between standard CTP parameters and the presence of any type of hemorrhage, HI1/HI2, and PH1/PH2, at follow-upa
Adjusted associations between standard CTP parameters and the presence of any hemorrhage, HI1/HI2, and PH1/PH2, at follow-upa
Hemorrhage Subtypes.
Both HI1/HI2 and PH1/PH2 groups differed with respect to rCBF <30% and CBV <38% compared with those without any hemorrhage (HI1/HI2: rCBF <30%; median, 15.5 mL [IQR: 5.9–47.7 mL] versus 6.1 mL [IQR: 0.0–22.5 mL]; P < . 001; CBV <38%; 17.8 [IQR: 4.9–44.2] versus 6.9 [IQR: 0.0–32.7]; P < . 001 and PH1/PH2: rCBF <30%; median, 19.9 mL [IQR, 6.9–32.8 mL] versus 6.1 mL [IQR, 0.0–22.5 mL]; P = . 001; CBV <38%; 16.3 [IQR, 6.7–45.4] versus 6.9 [IQR, 0.0–32.7]; P = .009). Larger CBF <30% volumes were observed in the PH1/PH2 group compared with the HI1/HI2 group, while CBV <38% deficit volumes were generally larger in the HI1/HI2 cohort compared with the PH1/PH2 group (Online Supplemental Data). These differences, however, were not significant (data not shown). No differences in Tmax > 6 seconds were observed for HI1/HI2 or PH1/PH2 (Online Supplemental Data). None of the tested CTP metrics differed according to presence of sICH (Online Supplemental Data).
For HI1/HI2, univariable regression analyses demonstrated a significant relationship between the rCBF <30% (OR, 1.18; 95% CI, 1.09–1.28; P < . 001, AUC, 0.65) and CBV <38% (OR, 1.10; 95% CI, 1.03–1.17; P = .004, AUC, 0.61) parameters, but not Tmax > 6 seconds (Table 1). After we adjusted for baseline, clinical, and procedural characteristics, however, neither relationship remained significant (Table 2).
When PH1/PH2 was taken as the dependent variable, univariable regression analysis revealed a significant relationship between rCBF <30% (OR, 1.15; 95% CI, 1.04–1.28; P = .007, AUC 0.64) (Table 1), which persisted following adjustment (Table 2). Neither univariable nor multivariable regression analyses showed significant associations between sICH and the CTP parameters (Online Supplemental Data).
No significant associations among any of the secondary CTP metrics, hypoperfusion-intensity ratio, mismatch, and mismatch ratio were observed (data not shown).
DISCUSSION
In the ESCAPE-NA1 trial, we found that higher volumes of rCBF <30% deficit (often operationally classified as “ischemic core”) were associated with the presence of any hemorrhage on follow-up imaging. However, this relationship is very likely driven by the association with PH1/PH2 hemorrhage subtype on 24-hour follow-up imaging, because no significant relationships were observed between CTP parameters and HI1/HI2 or sICH.
There is substantial heterogeneity in the literature, with studies reporting associations with prolonged Tmax14,15 and low CBV values,16,17 while others emphasized associations of low rCBF.18 Meta-analyses conducted on this topic have been limited by variations in perfusion metrics, software programs, and study designs (eg, indication, technique, and timing of follow-up imaging for hemorrhage detection).11,19⇓-21 Some have even identified a potential publication bias, suggesting an overestimation of the diagnostic performance of CTP for hemorrhage prediction.19
A few studies have specifically examined the associations of RAPID-generated CTP parameters. For instance, 1 study analyzed a cohort of 282 patients with (91 [32%]) and without (191 [68%]) hemorrhage at follow-up and found larger volumes of CTP parameters with hemorrhage.15 In this relatively small, single-center study, Tmax >6 was observed to be the strongest factor associated with hemorrhagic transformation. Another single-center study involving 392 patients undergoing EVT identified associations between ASPECTS and infarct core volume (defined by rCBF <30%), but the models were not adjusted for factors such as collaterals, blood pressure, or time to reperfusion, and the effect sizes were small.22
While larger rCBF <30% volumes demonstrated an association with the presence of parenchymal hematoma, no significant correlation was observed between CTP metrics and sICH. This finding may, in part, be due to the relatively low incidence of sICH in this cohort (14/408, 3.4%). Indeed, the overall trend was toward larger volumes in the sICH group (rCBF <30%: 15.9 versus 10.2 mL; CBV <38%: 30.3 versus 11.6 mL) (Online Supplemental Data). Most interesting, there was a trend toward smaller volumes of Tmax > 6 seconds in the symptomatic hemorrhage group (119.2 versus 137.7 mL) (Online Supplemental Data), potentially highlighting the importance of decreased mismatch volume. Nevertheless, this discrepancy prompts consideration of factors beyond perfusion imaging that might contribute to symptomatic hemorrhage post-EVT.
A strength of this study lies in its relatively large sample size derived from a randomized controlled trial, which may also explain the somewhat discrepant results regarding Tmax between the current study and other studies. Furthermore, the use of the same software and standardized output for all perfusion images enhances the consistency and clinical relevance of the findings. While other promising perfusion-based metrics, such as the permeability surface-area product, have been identified for hemorrhage prediction, their widespread use in clinical practice remains limited.11,23,24
Although certain baseline CTP parameters appear to be associated with hemorrhagic transformation at 24-hour follow-up, it is important to acknowledge that hemorrhage is a complex process influenced by multiple factors, many of which are not detectable through CTP imaging alone.
For instance, in addition to procedural factors such as treatment delays, complications, and reperfusion outcomes, previous studies have found associations of hemorrhagic transformation and hyperglycemia, acute hypertension, blood pressure variability, and stroke severity.5,6,25 The current study confirmed these associations with regard to any type of parenchymal hemorrhage and HI1/HI2, emphasizing the likely stronger association of these non-CTP variables with hemorrhage at follow-up. No such associations being seen in patients with PH1/PH2 might be attributed to the smaller sample size of patients with PH1/PH2, resulting in underpowered analyses.
Additionally, there may be information loss during postprocessing.26 By integrating clinical information with CTP, a more precise and individualized diagnostic framework for predicting hemorrhagic transformation could be achieved. However, larger studies are needed to further investigate these possibilities.
Limitations
Limitations of this study include those inherent to a randomized controlled trial, the heterogeneity introduced by batch-processing perfusion studies from different sites and machines, the reliance on the standard output parameters of RAPID without deeper analyses, and the use of both NCCT and MR imaging for assessing hemorrhagic transformation, all of which may have affected the precision of the estimates. Furthermore, without dual-energy CT, differentiating hemorrhage and contrast material staining on NCCT can be challenging. A study by Amans et al27 demonstrated that brain parenchyma with contrast staining on CT after DSA in patents with acute ischemic stroke was likely to infarct and unlikely to hemorrhage, suggesting that most contrast staining did not progress to hemorrhage. Although contrast extravasation occurs during the breakdown of the BBB, which also leads to bleeding, the volumes may have been overestimated and our results should be interpreted with caution. Finally, the grouping of different subtypes (HI1, HI2, PH1, PH2) may mask nuanced relationships. This grouping decision was influenced by the limited number of patients in each subgroup. While primarily PHs have been shown to have an impact on clinical outcomes, this reduction in granularity remains a limitation, and further studies with larger subgroup sizes would be valuable for a more detailed analysis.
CONCLUSIONS
This study demonstrates that larger volumes of rCBF <30% deficit are associated with an increased risk of developing PH1/PH2. However, no significant associations were found for HI1/HI2 or sICH. These findings suggest that while CBF <30% may help estimate the risk of more severe types of intracranial hemorrhage following EVT for acute ischemic stroke, other imaging, clinical, and procedural factors are likely of greater value.
Acknowledgments
We acknowledge the ESCAPE-NA1 sites and investigators.
Footnotes
Rosalie V. McDonough and Nathaniel B. Rex are co-first authors.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received November 14, 2023.
- Accepted after revision January 24, 2024.
- © 2024 by American Journal of Neuroradiology