Abstract
BACKGROUND: Antibodies against leucine-rich glioma inactivated protein 1 (LGI1) constitute a common form of autoimmune encephalitis. On MR imaging, it may show T2 FLAIR hyperintensities of the medial temporal lobe (T2 FLAIR-MTL), involve the basal ganglia, or be unremarkable.
PURPOSE: We performed a systematic review and meta-analysis to obtain prevalence estimates of abnormal findings on MR imaging in anti-LGI1 encephalitis. A human brain map of the LGI1 microarray gene expression was derived from the Allen Human Brain Atlas.
DATA SOURCES: PubMed and Web of Science were searched with the terms “LGI1” and “encephalitis” from inception to April 7, 2022.
STUDY SELECTION: Thirty-one research publications, encompassing case series and retrospective cohort and case-control studies, with >10 patients with anti-LGI1 encephalitis and MR imaging data were included.
DATA ANALYSIS: Pooled prevalence estimates were calculated using Freeman-Tukey double-arcsine transformation. Meta-analysis used DerSimonian and Laird random effects models.
DATA SYNTHESIS: Of 1318 patients in 30 studies, T2 FLAIR-MTL hyperintensities were present in 54% (95% CI, 0.48–0.60; I2 = 76%). Of 394 patients in 13 studies, 27% showed bilateral (95% CI, 0.19–0.36; I2 = 71%) and 24% unilateral T2 FLAIR-MTL abnormalities (95% CI, 0.17–0.32; I2 = 61%). Of 612 patients in 15 studies, basal ganglia abnormalities were present in 10% (95% CI, 0.06–0.15; I2 = 67%). LGI1 expression was highest in the amygdala, hippocampus, and caudate nucleus.
LIMITATIONS: Only part of the spectrum of MR imaging abnormalities in anti-LGI1 encephalitis could be included in a meta-analysis. MR imaging findings were not the main outcomes in most studies, limiting available information. I2 values ranged from 62% to 76%, representing moderate-to-large heterogeneity.
CONCLUSIONS: T2 FLAIR-MTL hyperintensities were present in around one-half of patients with anti-LGI1. The prevalence of unilateral and bilateral presentations was similar, suggesting unilaterality should raise the suspicion of this disease in the appropriate clinical context. Around 10% of patients showed basal ganglia abnormalities, indicating that special attention should be given to this region. LGI1 regional expression coincided with the most frequently reported abnormal findings on MR imaging. Regional specificity might be partially determined by expression levels of the target protein.
ABBREVIATIONS:
- ADAM
- a disintegrin and metalloprotease domain
- AIE
- autoimmune encephalitis
- AMPA
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- BG
- basal ganglia
- CASPR2
- contactin-associated protein-like 2
- EEG
- electroencephalogram
- FBDS
- faciobrachial dystonic seizures
- LGI1
- leucine-rich glioma inactivated protein 1
- MTL
- medial temporal lobe
Antibodies against leucine-rich glioma inactivated protein 1 (LGI1) characterize the second most common form of autoimmune encephalitis (AIE).1 These antibodies were first described in 2010 and were previously clustered within voltage-gated potassium channel antibodies, which included contactin-associated protein-like 2 (CASPR2) along with LGI1.2 The LGI1 protein is widely expressed in the CNS and particularly in neurons, where it forms a trans-synaptic protein complex that regulates ion channel and synaptic activity.3,4
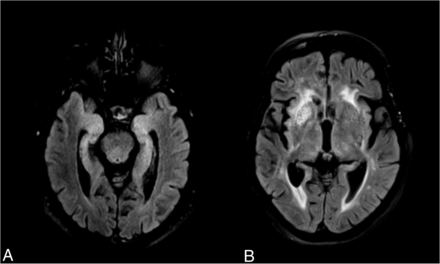
This disease typically presents with subacute cognitive impairment and epilepsy, but faciobrachial dystonic seizures (FBDS) are the most distinctive clinical feature. Although presenting across a wide variety of ages, it is thought to be most common in those older than 60 years of age.3 The clinical presentation thus leads to a vast differential diagnosis, in which clinical suspicion plays an important role.5,6 MR imaging is relevant in supporting the diagnosis of AIE and excluding alternative diagnoses. Characteristically, it involves medial temporal lobe (MTL) hyperintensities on T2 FLAIR sequences, which represent a criterion for a definite diagnosis of AIE when observed bilaterally (Fig 1A).7,8 This laterality issue is because in the absence of antibody confirmation, unilateral hippocampal/MTL abnormalities have a wider radiologic differential diagnosis.7,9 The main diagnostic considerations in this imaging presentation include seizure-related MR imaging abnormalities;10 viral encephalitis, particularly herpes simplex virus 1 and 2 and human herpesvirus 6;11 neurosyphilis;12 Whipple disease,13 temporal lobe glioma,14 and acute posterior circulation ischemic infarction.15
Illustrative examples of signal abnormalities in 2 cases of confirmed anti-LGI1 encephalitis, one with medial temporal lobe T2-FLAIR hyperintensities (A) and another with right BG T2-FLAIR hyperintensity (B).
However, not all cases of anti-LGI1 encephalitis present with MTL abnormalities at the time of MR imaging. MR imaging can also be unremarkable or show basal ganglia (BG) involvement (Fig 1B).16 Moreover, given the relatively recent description of the wide range of antibodies causing AIE, MR imaging findings are not yet thoroughly described for each antibody group.8,17 It is, therefore, important to provide prevalence estimates of MR imaging abnormalities in the literature for each type of antibody-mediated encephalitis. Indeed, given the wide clinical and brain imaging differential diagnoses involved in these cases, detailing the prevalence of specific patterns of abnormal findings on MR imaging for each encephalitis might aid the radiologist in providing a narrower differential while waiting for laboratory results.
Furthermore, abnormalities on MR imaging are likely reflective of pathophysiologic mechanisms. Characterizing the topography of the most frequently reported abnormalities may shed light on the underlying causative pathology. Although most studies have focused on LGI1 expression in rodent models derived from immunohistochemistry or patient-derived antibodies,3,4 a human brain map of the LGI1 gene might reveal why selective brain areas are impacted in this disease.
In this study, we performed a systematic review and meta-analysis of the proportion of MTL and BG MR imaging signal abnormalities in LGI1 encephalitis. Moreover, we used the publicly accessible Allen Human Brain Atlas (https://human.brain-map.org) to derive a LGI1 gene expression map of the human brain, which can provide potential hints as to why LGI1 encephalitis preferentially affects certain brain regions.
MATERIALS AND METHODS
This systematic review and meta-analysis was conducted in accordance with the PRISMA guidelines.18
Search Strategy and Selection Criteria
We searched PubMed and the Web of Science from inception to April 7, 2022, using the search terms “LGI1” and “encephalitis.” PubMed had 511 publications and the Web of Science had 646 publications. Bibliographic sources from selected articles were also used to assess research publications for eligibility. Original research articles, including case series, retrospective or prospective cohort studies, and case-control studies were considered for selection. Case reports, systematic reviews, meta-analyses, review articles, and other nonoriginal research publications were excluded during screening. Only studies conducted with human participants and published in English were considered. The number of each article type found during screening is provided in the Online Supplemental Data.
Inclusion and Exclusion Criteria
Research publications were eligible if they included a clinical population of LGI1 encephalitis and reported >10 cases, when revealed in the abstract. The clinical definition of LGI1 encephalitis was then confirmed on inspection of the publications to comply with definite autoimmune encephalitis, requiring detection of anti-LGI1 antibodies on CSF and/or serum.7 Exclusion criteria after retrieval were the following: the study did not present MR imaging data, did not clearly specify the MR imaging abnormalities/brain location/MR imaging sequence used, did not use T2-FLAIR sequences or used other types of MR imaging data not widely used in clinical practice, had ≤10 patients with LGI1 encephalitis, did not separate LGI1 from CASPR2 encephalitis or from other autoantibodies; and the data were previously published in other research publications, there was no accessibility of the full text, or the article had low manuscript quality.
Data Extraction
Two authors independently screened titles and abstracts and assessed the full text for eligibility (F.C.A., A.I.P). Discrepancies were solved after discussion between the 2 authors. Clinical data (J.L., J.M.), electroencephalogram (EEG) data (G.V., F.C.A.), and neuroimaging data (F.C.A., C.M.-P.) were extracted independently by 2 authors. When necessary, discrepancies were solved by a third party (clinical: R.S.; neuroimaging: T.G.O.). The quality of the studies was assessed independently by 2 authors (F.C.A. and A.I.P.) on the basis of a previously published tool for prevalence studies.19 This tool consists of 10 items consisting of 4 external validity and 6 internal validity items, the sum score of which provides a summary assessment of the risk of bias.
Data Analysis
Pooled prevalence estimates of neuroimaging findings were calculated using Freeman-Tukey double-arcsine transformation. Meta-analysis was conducted using DerSimonian and Laird random-effects models. Heterogeneity was calculated using I2 statistics, and the Cochran Q test was used to assess statistical significance. The metaprop package in R (https://www.rdocumentation.org/packages/meta/versions/6.2-1/topics/metaprop) was used for this analysis.
Metaregression analysis was conducted to assess modifiers of prevalence. Clinical symptoms and CSF antibody positivity were used in univariate models to assess the effects on the prevalence of both T2 MTL hyperintensities and BG signal abnormalities (T2 hyperintensities and T1 hypo- and hyperintensities). Studies with missing data on modifier variables were excluded. R2 was used to assess the proportion of the heterogeneity explained by the modifier, the P value of the Quantitative Methods statistics was used for statistical significance, and I2, for residual heterogeneity left unexplained. The Meta package in R was used for this analysis (https://cran.r-project.org/web/packages/meta/index.html).
Publication bias was assessed with visual inspection of funnel plots and assessed for statistical significance using the Egger weighted linear regression test. The meta package in R was used for this analysis.
LGI1 Gene Brain-Expression Map
Regional microarray expression data were obtained from 6 postmortem brains (1 woman; age range, 24.0–57.0 years; mean age, 42.50 [SD, 13.38] years) provided by the Allen Human Brain Atlas.20 Data were processed with the abagen toolbox (version 0.1.3; https://github.com/rmarkello/abagen) using an 83-region volumetric atlas in Montreal Neurological Institute space. First, microarray probes were reannotated using data provided by Arnatkeviciute et al.21 Probes not matched to a valid Entrez ID were discarded. Next, probes were filtered on the basis of their expression intensity relative to background noise22 so that probes with intensity less than the background in ≥50% of samples across donors were discarded, yielding 31,569 probes. When multiple probes indexed the expression of the same gene, we selected and used the probe with the most consistent pattern of regional variation across donors (ie, differential stability23). Here, regions correspond to the structural designations provided in the ontology from the Allen Human Brain Atlas. The Montreal Neurological Institute coordinates of tissue samples were updated to those generated via nonlinear registration using the Advanced Normalization Tools (ANTs; http://stnava.github.io/ANTs/). Samples were assigned to brain regions in the provided atlas if their Montreal Neurological Institute coordinates were within 2 mm of a given parcel.
To reduce the potential for misassignment, sample-to-region matching was constrained by hemisphere and gross structural divisions (ie, cortex, subcortex/brainstem, and cerebellum, such that for example, a sample in the left cortex could only be assigned to an atlas parcel in the left cortex21). All tissue samples not assigned to a brain region in the provided atlas were discarded. Intersubject variation was addressed by normalizing tissue sample expression values across genes using a robust sigmoid function.24 Normalized expression values were then rescaled to the unit interval. Gene expression values were then normalized across tissue samples using an identical procedure. Samples assigned to the same brain region were averaged separately for each donor and then across donors, yielding a regional expression matrix with 83 rows corresponding to brain regions and 15,633 columns corresponding to the retained genes. Normalized gene expression values were then plotted in a brain map using the ggseg R package (https://cran.r-project.org/web/packages/ggseg/index.html)25 with a Desikan-Killiany atlas.
Data Availability
Data used in this meta-analysis will be provided at request.
RESULTS
Study and Clinical Characteristics
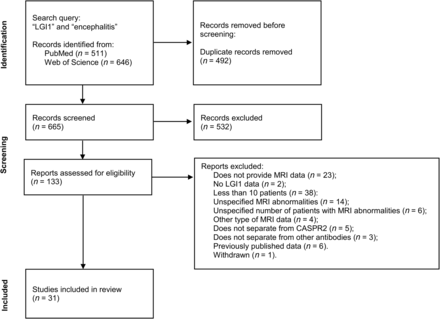
In this study, we included 31 research publications (Fig 2).5,16,26-54 Two studies reported partially on the same patients but focused on different neuroimaging findings.16,50 For summary statistics regarding each of the clinical and neuroimaging findings, we excluded the publication with fewer patients. In total, the sample was composed of 1409 patients with anti-LGI1 encephalitis. The mean quality-assessment score of the research publications was 3, indicating overall good quality (Online Supplemental Data).
Study selection flow chart.18 PubMed and Web of Science were searched for the terms “LGI1” and “encephalitis.” Of 665 records screened, 133 were assessed for eligibility, resulting in 31 included publications.
Most of the studies were retrospective and based on clinical cohorts, with some studies drawing their sample from EEG or PET cohorts. Case definition was mostly based on criteria for definite AIE,7 with 3 studies basing their definition on FBDS and LGI1 antibody positivity.16,43,49
Table 1 reports summary statistics for the whole study population. The mean age was 61.4 years, and 64% of patients were men. Seventy-eight percent of the patients presented with cognitive impairment (1095 of 1409). For FBDS, 27 studies presented data, totaling 1252 patients, among whom 639 (51%) had FBDS at any point in time. Twenty-eight studies reported seizures other than FBDS, totaling 1368 patients, of whom 989 (72%) were affected. Twenty-four studies, encompassing 1146 patients included data on hyponatremia, among whom 512 (45%) patients presented with low serum sodium. Consciousness impairment was evaluated in 12 studies with 574 patients and was present in 180 (31%). Autonomic dysfunction was present in 155 patients of 812 (19%) in 15 studies. Sleep disorder was present in 417 patients of 1166 (36%) in the 21 studies that reported data on this symptom. Of 1203 patients, 612 in 25 studies presented with psychiatric symptoms (51%). Hallucinations were present in 80 of 493 patients (16%) in 9 studies. Personality changes and psychosis (unspecified) were among other symptoms reported in the included literature. In 14 studies with 713 patients, CSF antibody positivity was found in 457 (64%). In 19 studies with data on EEG, 508 of 698 patients (73%) presented with abnormal findings.
Summary statistics for the whole study population
Neuroimaging Findings
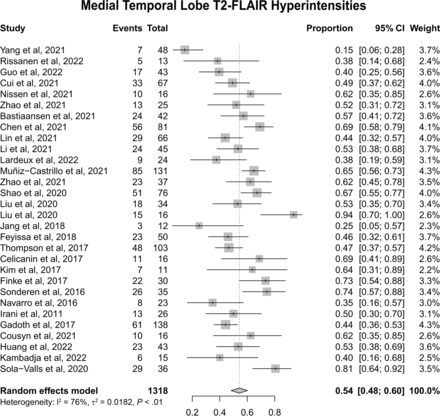
Of the whole sample of 1409 patients, 1318 had reported data on MR imaging. Thirty studies reported data on T2 or T2 FLAIR-MTL hyperintensities. Meta-analysis of the proportion of patients with T2 FLAIR-MTL revealed that this finding was present in 54% of patients (Fig 3; 95% CI, 0.48–0.60; I2 = 76%). Thirteen studies reported T2 FLAIR-MTL laterality with a total of 394 patients. Meta-analysis revealed 27% with bilateral T2-MTL findings (Fig 4A; 95% CI, 0.19–0.36; I2 = 71%) and 24% with unilateral abnormalities (Fig 4B; 95% CI, 0.17–0.32; I2 = 61%) of the whole population (ie, with and without T2-MTL abnormalities).
Forest plot showing the prevalence of T2 FLAIR-MTL hyperintensities. Of 1318 patients in 30 studies, 54% presented with this signal abnormality.
Forest plots showing the prevalence of bilateral T2 FLAIR-MTL hyperintensities (27% of 394 patients in 13 studies) (A) and unilateral MTL T2 FLAIR-MTL hyperintensities (24% of 394 patients in 13 studies) (B).
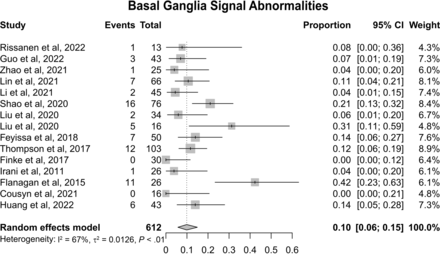
Fifteen studies encompassing 612 patients reported data on BG MR imaging signal abnormalities, which included T2 hyperintensities and T1 hypo- and hyperintensities. Altogether, meta-analysis of proportions revealed these signal abnormalities to be present in 10% of patients (Fig 5; 95% CI, 0.06–0.15; I2 = 67%).
Forest plot showing the prevalence of BG signal abnormalities (T2-FLAIR hyperintensities and T1 hypo- and hyperintensities with 10% of 612 patients having these abnormalities).
Inspection of funnel plots and linear regression testing for funnel asymmetry did not reveal a significant potential for publication bias (Online Supplemental Data).
Although not reported frequently enough to undergo meta-analysis, studies showed anti-LGI1 encephalitis with other regional and MR abnormalities.26,-,38,40,41,43,45⇓⇓-48,51,54 Amygdala, insula, and other neocortical T2-FLAIR hyperintensities were found in around 10%, 8%, and 18% of patients, respectively (Table 2). Two patients presented with cortical diffusion restriction (2%). Leptomeningeal gadolinium enhancement was described in 3 patients (5%). MTL swelling was reported in 18 patients (15%), and atrophy, in 55 patients (16%), whereas gadolinium enhancement was found in 5 patients (3%). In the studies with available longitudinal follow-up, 46% of patients of 154 in 6 studies developed atrophy or mesial temporal sclerosis. BG atrophy was reported in 3 patients (3%). Neocortical and cerebellum gadolinium enhancement was described in 1 patient each in 1 study.27 Diffusion restriction in the basal ganglia was reported in 1 study.16
Other MR imaging abnormalities associated with anti-LGI1 encephalitis
Given the high heterogeneity found in the meta-analysis of these neuroimaging findings, we performed meta-regression analysis for T2-MTL and BG signal abnormalities with clinical predictors (Online Supplemental Data). We did not find any statistically significant modifiers of the proportion of MR imaging signal abnormalities.
LGI1 Gene Expression Map of the Human Brain
To assess whether the LGI1 gene is differentially expressed in the selective regions affected on MR imaging, we next used publicly available data from the Allen Human Brain Atlas to create a gene-expression brain map. We found that LGI1 is expressed widely across the cortex but is particularly predominant in subcortical regions, namely, the amygdala, hippocampus, and caudate nucleus (Fig 6A,-B; and Online Supplemental Data).
LGI1 gene expression in the cortical (A) and subcortical (B) regions derived from the Allen Human Brain Atlas. In this figure, LGI1 gene expression is shown to be higher in the MTLs and BG, concordant with the main MR imaging signal abnormality findings, suggesting that regional brain susceptibility in anti-LGI1 encephalitis might be mediated by the relative levels of brain regional expression of this gene.
DISCUSSION
In this systematic review and meta-analysis, we focused on neuroimaging findings of anti-LGI1 encephalitis. We included 31 studies and 1409 patients. Our studied population had a mean age of 61.4 years and a mild predominance of male patients (64%). The most frequently reported symptoms were cognitive impairment, seizures other than FBDS, followed by FBDS and psychiatric symptoms. These clinical and demographic characteristics are in agreement with those in previous studies.55
We found that 54% of patients presented with T2-FLAIR hyperintensities of the MTL (Fig 3). This finding reinforces the idea that a negative MR imaging in anti-LGI1 encephalitis is frequent. In a subgroup analysis, unilateral and bilateral presentations were similar, with a prevalence of 24% and 27%, respectively (Fig 4A, -B). This finding suggests that in the appropriate clinical context, the presence of unilateral MTL abnormalities on MR imaging should raise the possibility of AIE, namely anti-LGI1 encephalitis, and may aid in therapeutic decisions while awaiting antibody confirmation. A common imaging differential diagnosis for T2 FLAIR-MTL hyperintensities frequently associated with volume expansion includes other autoimmune/paraneoplastic encephalitis cases, among which the neuronal-surface antigen group includes NMDAR,56 CASPR2,57,58 GABA-B,59,60 Neurexin-3a,61 AMPAR,62,63 mGluR5,64 GlyR,65,66 AK5,67 and DPPX6,68 and in the intracellular antigen group includes GAD65,69 Hu,8 amphiphysin,70 and Ma2.71 Viral encephalitis,11 neurosyphilis,12 Whipple disease,13 glioma,14 ictal changes,9 and posterior circulation stroke15 are among other important diagnostic considerations. Depending on the time of imaging, anti-LGI1 encephalitis might already have presented with hippocampal atrophy, in which case neurodegenerative diseases, including Alzheimer disease,72,73 frontotemporal dementia,74 and limbic-predominant age-related TDP-43 encephalopathy,75 as well as medial temporal sclerosis of other causes, are to be included in the differential diagnosis. Other causes to consider, depending on clinical presentation and other associated signal changes, include metabolic and mitochondrial disorders.76
Notably, BG signal abnormalities, encompassing both T2 and T1 hyperintensities and T1 hypointensities, were reported in around 10% of patients (Fig 5). The available number of studies providing information regarding the BG was substantially smaller than that for the MTL. However, these changes can be the sole signal alteration observed on MR imaging and might be unilateral16 (Fig 1B), suggesting that this brain region deserves detailed attention when suspecting AIE. In fact, LGI1 expression is high in the BG in humans (Fig 6B), and [18F] fluorodeoxyglucose PET studies have repeatedly shown metabolic alterations in these regions.27,39,41 Most important, these lesions can present with diffusion restriction,16 the cause of which is unknown. Ischemic infarction is thus an important differential diagnosis, and attention to clinical presentation is crucial.
The nature of T1 hyperintensities of the BG is also not yet understood. Underlying causes of T1 relaxation time shortening included blood, fat, high paramagnetic substances such as iron, and high protein content. In anti-LGI1 encephalitis, BG T1-hyperintensities are sometimes preceded by T2-hyperintensities and have been shown to remain for a longer time than the latter before resolving.16 A putative explanation might be the accumulation of proteins at the site of antibody engagement, which persists after initial inflammatory changes. A parallel explanation has been drawn for T1 pallidal hyperintensities in Creutzfeldt-Jakob disease, where accumulation of prion protein, confirmed at neuropathology, was correlated with this MR imaging signal abnormality.77 Signal alterations in the BG have a vast differential diagnosis, which includes toxic and metabolic disorders,78 among which hepatic failure and Wilson disease are important considerations, Creutzfledt-Jakob disease,79 other autoimmune encephalitis (eg, CV2/collapsin response mediated protein 5),80 viral encephalitis,11 and arterial and venous infarction, among others.81
Other brain regions were found to be altered in LGI1 encephalitis, namely, the amygdala, insula, cerebellum, and different regions of the neocortex, with signal abnormalities across T1 with gadolinium enhancement, T2 and diffusion MR imaging, as well as leptomeningeal gadolinium enhancement. This finding suggests that LGI1 AIE can present with a widespread variety of signal abnormalities across multiple brain regions and multiple MR imaging sequences. Moreover, the under-reporting of altered signal beyond the MTL indicates that there is a need for further research in systematizing MR imaging signal abnormalities in LGI1 encephalitis across the whole brain.
In the studies that included a follow-up period, 46% of patients went on to develop hippocampal atrophy or mesial temporal sclerosis (Table 2). These results support the concept that anti-LGI1 encephalitis might induce a neurodegenerative process in a large proportion of patients, which might be associated with remaining cognitive deficits.82 Detection of these imaging findings by the radiologist in serial scans provides useful information for the clinician.
To assess qualitatively whether MR imaging abnormalities tend to occur at brain regions most enriched for LGI1 gene expression, we generated a brain map using the publicly available Allen Human Brain Atlas. Regions with the highest expression were the amygdala, hippocampus, and caudate (Fig 6), suggesting that at least partially, the selective appearance of abnormalities on MR imaging might be due to the relative expression of LGI1. The LGI1 protein is expressed at the synapse, where binding presynaptic a disintegrin and metalloprotease domain 23 (ADAM23) and postsynaptic ADAM22 forms a trans-synaptic tether that couples presynaptic voltage-gated potassium channels and postsynaptic AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors, thereby regulating synaptic activity.3 Evidence from animal models has shown that patient-derived anti-LGI1 antibodies induce important impairment of synaptic plasticity,83,84 neuronal excitability85 and memory in rodents.83,84 This evidence supports the pathogenicity of the antibody and the hypothesis that higher expression of the target antigen might be a contributing factor to explain brain regional susceptibility in these diseases. The accessibility of the antibodies to these brain regions is also a putative contributing factor. Many patients presenting without MR imaging signal abnormalities suggests that only in certain cases is the underlying pathophysiologic mechanism capable of generating a detectable change on MR imaging. The reasons are still to be explored. PET imaging approaches might unravel promising biologic markers of disease activity in the acute phase.86
While these results provide the first estimate of neuroimaging signal abnormalities reported in the literature for LGI1 encephalitis, this study has important limitations. Even though MR imaging signal abnormality is likely to be present at the time of symptom onset, we were unable to report any analysis on this parameter. Moreover, neuroimaging findings were not the main outcome in most studies. While we did not find any clinical modifiers on the prevalence of these neuroimaging findings, not all studies provided data for all subjects who underwent MR imaging. More research is needed to establish whether signal abnormalities on MR imaging correlate with clinical presentation or other auxiliary tests. Finally, the specific MR imaging sequence parameters and magnetic field strength of the scanner were not taken into account in the analyses.
Most important, a considerable number of studies were excluded due to incomplete reporting on MR imaging data (Fig 2). These results lead us to highlight the need to include detailed MR imaging descriptions in a disease that can present with a variety of signal abnormalities across various MR imaging sequences.
CONCLUSIONS
This study presents a first approach to estimate the main findings of anti-LGI1 AIE on MR imaging and underlines the importance of considering this diagnosis in the context of unilateral signal abnormalities of the MTL, while also calling for detailed attention to the BG with suspicion of this disease. Most interesting, these regions express the LGI1 gene at high levels, thus lending support to the hypothesis that the regions affected by autoimmunity might be at least partially determined by the expression levels of the target protein.
Acknowledgments
We thank Dr Alexandra Rodrigues from the Neuroradiology Department of Centro Hospitalar Universitário de São José for her valuable input.
Footnotes
↵† Raquel Samões and Tiago Gil Oliveira contributed equally to this work.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.
- 43.↵
- 44.
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.
- 53.
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- Received November 14, 2023.
- Accepted after revision February 14, 2024.
- © 2024 by American Journal of Neuroradiology