Abstract
BACKGROUND AND PURPOSE: Pathologic studies suggest that neovascularization and hemorrhage are important features of plaque vulnerability for disruption. Our aim was to determine the associations of these features in carotid plaques with previous cerebrovascular ischemic events by using high-resolution CE-MRI.
MATERIALS AND METHODS: Forty-seven patients (36 men; mean age 72.5 ± 10 years) underwent CE-MRI and MRA examinations for carotid plaque at 3T. IPH presence was recorded. Neovascularity was categorized by the degree of adventitial enhancement (0, absent; 1, <50%; 2, ≥50%). Reader variability was assessed by using weighted κ. Associations with events were determined by using multivariable logistic regression.
RESULTS: Intra- and inter-reader agreement for grading adventitial enhancement were good to excellent. IPH was present in 49% of patients and was associated with events (P = .03). Patients grouped by categories 0, 1, and 2 adventitial enhancement had increasing frequencies of events (14% category 0, 48% category 1, 65% category 2; P = .02). Events were associated with IPH (OR, 10.18; 95% CI, 1.42–72.21) and adventitial enhancement (compared with category 0: OR, 14.90, 95% CI, 0.98–225.93 for category 1; OR, 51.17, 95% CI, 3.4–469.8 for category 2) after controlling for age, sex, cardiovascular risk factors, wall thickness, and stenosis. Stenosis was not associated with events.
CONCLUSIONS: Adventitial enhancement and IPH are independently associated with previous events and may provide important insight into stroke risk not achievable by stenosis.
ABBREVIATIONS:
- BBMRI
- black-blood MR imaging
- CE
- contrast-enhanced
- CI
- confidence interval
- ICC
- intraclass correlation coefficient
- IPH
- intraplaque hemorrhage
- OR
- odds ratio
- TOF
- time-of-flight
There is increasing evidence that atherosclerotic plaque components and not simply the stenosis caused by plaque are important determinants of clinical events including acute coronary syndrome, TIA, and stroke.1 Recent work has established a central role for neovascularity and IPH in the initiation, progression, and rupture of atherosclerotic plaques.2⇓–4 Both pathologies are thought to be intimately involved in plaque progression and disruption. IPH, which develops from the rupture of incompetent neovessels,5 leads to the formation of the thrombogenic lipid core.4 Both IPH and neovascularity contribute to inflammatory cell recruitment and plaque instability.3,4 Finally, their role in plaque disruption is supported by specimen studies that have identified greater IPH6 and neovasculization7 in symptomatic compared with asymptomatic plaques.
High-resolution MR imaging allows accurate characterization of plaque components such as IPH. Moreover, gadolinium contrast administration improves plaque characterization8,9 and enables identification of neovascularity.10 Adventitial enhancement, in particular, serves as a useful marker of neovascularization11,12 because the adventitia is rich in vasa vasorum, the main source of intimal neovessels.4
Although neovascularization and IPH are thought to be important markers of vulnerability, their combined role in plaque rupture has not been studied in vivo, to our knowledge. Our aim was to identify these features by using high-resolution MR imaging and to determine their associations with recent cerebrovascular ischemic events.
Materials and Methods
Study Population
Participants for this study were drawn from patients who underwent high-resolution CE-MRA/CE-MRI for carotid plaque evaluation. Reasons for patient referral for this assessment included the following: 1) validating of stenosis measurements initially made by an MRA performed elsewhere or by CT angiography or sonography, and 2) defining any potentially sinister features of a known carotid plaque (eg, ulceration, IPH). Patients were deemed symptomatic in this study if they had a recent TIA, stroke, or amaurosis fugax ipsilateral to the carotid artery of interest, with a target interval of ≤12 weeks preceding the MR imaging.13 Asymptomatic patients were referred because of either incidental carotid bruits or stenosis detected on routine sonography screening in the course of other investigations such as for cardiac clearance. Patients who had a carotid endarterectomy on the side to be studied by MR imaging were excluded. A total of 49 participants were scanned. Institutional review board approval was obtained to build the data base from which statistics were generated in this study, and research participants provided informed consent.
CE-MRI
All participants underwent a high-resolution CE-MRI examination on a 3T MR imaging scanner (Achieva; Philips Healthcare, Best, the Netherlands) by using either a 4-channel (Pathway MRI, Seattle, Washington; n = 14) or an 8-channel14 (n = 35) phased-array carotid coil. A standard MR imaging protocol was used for all examinations, which included pre- and postcontrast T1-weighted BBMRI, 3D TOF MRA, and 3D CE-MRA sequences. The 3D TOF sequence was used to localize the carotid bifurcations, and the BBMRI images were acquired by using a 2D electrocardiogram-gated double inversion recovery fast spin-echo sequence (TR/echo train length/TE, 1 RR/10/9 ms; 1 excitation; section thickness, 2 mm with 0 gap; acquired resolution, 0.35 × 0.35 × 2 mm). The TI was set to suppress the signal intensity of flowing blood. Sections were oriented perpendicular to the plaque and centered at the thickest part, with enough acquired to cover the entire plaque (≥5 sections). A 3D CE-MRA was then acquired before (ie, mask) and after (ie, during arterial phase) the intravenous injection of 0.05-mmol/kg (n = 10) or 0.1 mmol/kg (n = 39) gadopentetate dimeglumine (Magnevist; Schering, Berlin, Germany) by using a power injector (acquired resolution, 0.66 × 0.66 × 1 mm3). The BBMRI images were repeated 5 minutes after contrast administration. The TI was adjusted to account for contrast (250 ms).
MR Imaging Analysis
Two experienced readers (Y.Q. and M.E.), who were blinded to the characteristics of the study population, independently analyzed the MR images. The readers used semiautomated software (VesselMASS; Leiden University Medical Center, the Netherlands) to generate wall thickness measurements on the precontrast BBMRI section showing the thickest plaque. Precontrast images were used to avoid the inclusion of adventitial enhancement in the thickness measurement. First, edge-enhanced (gradient) images were generated from the original gray-scale images by using Sobel operator implemented in VesselMASS15 to eliminate the influence of subjective window/level settings for vessel contour detection. Lumen and outer wall contours were drawn by using the gradient image, and mean and maximum wall thickness values were generated.
Only those cases with a maximum wall thickness ≥1.5 mm were evaluated. This constraint was designed to ensure visual discrimination of vessel wall components at the acquired resolution (0.35 × 0.35 mm2 in-plane) by requiring ≥4 pixels across the wall, as reported previously.16
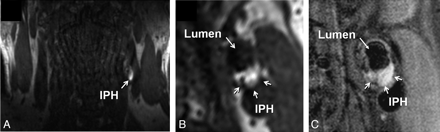
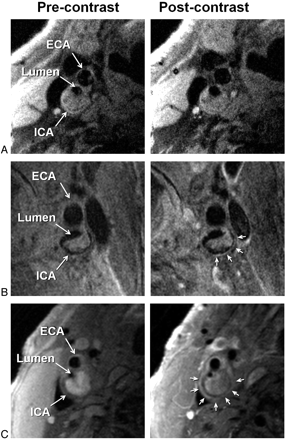
IPH was identified, by using established criteria, as hyperintense on heavily T1-weighted gradient-echo images (ie, CE-MRA mask images) (Fig 1).17,18 Adventitial enhancement was categorized as a marker of neovascularity on the basis of postcontrast BBMRI images by using the corresponding precontrast series for reference. Adventitial enhancement categories were determined by visual estimation of the percentage of involvement of the outer wall circumference (ie, adventitia) for the section showing the thickest plaque as follows: 0, no enhancement; 1, enhancement of <50% of the outer wall circumference; and 2, enhancement of ≥50% of the outer wall circumference (Fig 2). Carotid stenosis was measured by an attending neuroradiologist (B.A.W.) according to the NASCET guidelines based on the TOF MRA.19,20
Identification of IPH in the left carotid artery of a 60-year-old man with an ipsilateral cerebrovascular ischemic event. A, Source image from the coronally oriented CE-MRA mask sequence shows hyperintense signal intensity in the left carotid artery wall corresponding to IPH. The high signal intensity is seen on the transverse view of the mask image (B) reconstructed in the same plane as the T1-weighted precontrast BBMRI (C).
Examples of adventitial enhancement categories based on BBMRI acquired after the intravenous administration of gadolinium-based contrast (right column) by using the corresponding precontrast images for reference (left column). Categories 0, 1, and 2 are shown with no enhancement (A), <50% enhancement (B), and ≥50% enhancement (C) of the adventitia (arrows), respectively. ICA and ECA indicate internal carotid artery and external carotid artery, respectively.
Statistical Analysis
Data were analyzed by using the Statistical Package for the Social Sciences, Version 17.0 (SPSS RR, Chicago, Illinois) and STATA 10.0 (StataCorp, College Station, Texas). Continuous variables were compared by using either Student t tests or Mann-Whitney nonparametric tests. Categoric variables were compared by using χ2 tests. Robust variance estimates were used to account for repeated measurements within subjects. Multivariable logistic regression was used to identify predictors of stroke/TIA from the following variables: IPH, adventitial enhancement, carotid stenosis, maximum wall thickness, risk factors (smoking, diabetes, hypertension, and hyperlipidemia), statin use, and aspirin use. Wall thickness was included in the model to assure that the results for other variables pertain to their relation with stroke/TIA and not just their association with vessel size. The regression models included random effects for different MR imaging coils and gadolinium doses. Inter- and intrareader variability was assessed by using the ICC for continuous variables and Cohen weighted κ for categoric variables. Reliabilities <0.4 were characterized as poor; 0.4–0.75, as fair to good; and >0.75, as excellent.21
Results
Study Population Characteristics
Among the 49 patients, 2 were excluded from analysis. One patient could not tolerate the entire MR imaging examination, and 1 was discovered to have a dysplastic carotid artery rather than an atherosclerotic lesion. None of the remaining 47 patients were excluded for having a maximum wall thickness <1.5 mm. Among the 47 patients, 36 were men; 43, white; 2, African-American; 2, Asian; and the mean age was 72.5 ± 10.0 years. Twenty-four were symptomatic (ipsilateral anterior circulation stroke15 or TIA9) and 23 were asymptomatic. The median interval between symptom onset and MR imaging was 56 days (interquartile range, 41–65 days). The clinical characteristics of the study population are shown in Table 1. Carotid stenosis ranged from 0% (ie, carotid bulb lesions) to 99% (mean, 58.5%). Mean and maximum wall thickness measurements were similar between symptomatic versus asymptomatic individuals (2.71 ± 0.70 mm versus 2.63 ± 0.79 mm, P = .64, and 4.82 ± 1.14 mm versus 4.71 ± 1.61 mm, P = .62, respectively).
Demographic and clinical characteristics of participantsa
MR Imaging Measurement Reliability
Intra- and inter-reader agreement for grading adventitial enhancement at the thickest section was good to excellent (κ = 0.73 [95% CI, 0.41–0.99] and κ = 0.78 [95% CI, 0.58–0.98], respectively). The readers never disagreed by >1 category. Intra- and inter-reader agreement for IPH detection was excellent (κ = 0.97 [95% CI, 0. 89–1] and κ = 0.96 [95% CI, 0. 92–0.98], respectively). Inter-reader agreement for mean and maximum wall thickness measurements was excellent (ICC, 0.95 [95% CI, 0.92–0.97] and ICC, 0.94 [95% CI, 0.90–0.97], respectively).
Characteristics of Patients with Adventitial Enhancement and IPH
Each reader identified 40 of 47 (85%) patients as having adventitial enhancement (category 1 or 2). Characteristics of patients grouped by enhancement category by reader 1 are shown in Table 2. There was no association between adventitial enhancement categorized by either reader and cardiovascular risk factors, statin or aspirin use, maximum wall thickness, or stenosis for either reader. Patients grouped by categories 0, 1, and 2 of adventitial enhancement had increasing frequencies of prior ischemic events (14% category 0, 48% category 1, 65% category 2; P = .02, based on combined data from readers 1 and 2).
Characteristics of participants grouped by adventitial enhancement category and IPH presencea
Reader 1 identified 22 and reader 2 identified 24 of 47 (average, 49%) patients as having IPH, which was more prevalent in men. Characteristics of patients grouped by IPH detected by reader 1 are shown in Table 2. IPH identified by either reader was not associated with cardiovascular risk factors, statin use, or aspirin use but was associated with greater stenosis, maximum wall thickness, and ischemic events.
Associations with Cerebrovascular Ischemic Events
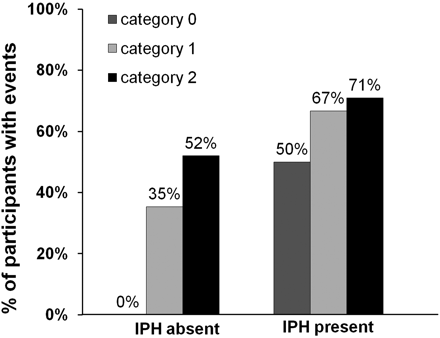
Because IPH is associated with ischemic events and neovascularity is thought to lead to IPH, we stratified patients with adventitial enhancement into those with and without IPH to determine whether the association of adventitial enhancement with ischemic events was mediated through IPH (Fig 3). Even in the absence of IPH, the adventitial enhancement category correlated with a higher ischemic event rate (P = .01). When both IPH and neovascularization were present, the ischemic event rate was highest (71%) compared with patients with none or only 1 of these features.
The relation between adventitial enhancement and prior cerebrovascular ischemic events stratified for patients with and without IPH present.
The independent associations of adventitial enhancement category, IPH presence, wall thickness, stenosis, statin and aspirin use, and demographic and risk factors with prior cerebrovascular events are shown in Table 3. Events were associated with the presence of IPH (OR, 10.18; 95% CI, 1.42–72.21) and adventitial enhancement (OR, 14.90; 95% CI, 0.98–225.93 for category 1; OR, 51.17; 95% CI, 3.4–469.80 for category 2), controlling for differences in coils and gadolinium doses. However, there was no association between stenosis and ischemic events (OR, 0.05; 95% CI, 0.02–1.50) or between maximum wall thickness and events (OR, 0.77; 95% CI, 0.40–1.45).
Multivariable analysis for participants with previous cerebrovascular ischemic eventsa
Discussion
We have shown that neovascularity and IPH presence in carotid plaque are independently associated with previous cerebrovascular events. The association with neovascularity as measured by adventitial enhancement remained strong even after adjusting for statin use, a potential inhibitor of plaque inflammation.22 These features might serve as individual markers of plaque instability, and their combined presence might be particularly alarming because this was seen in the group with the highest frequency of events. Of note, these associations with events could not be explained by arterial stenosis or wall thickness, highlighting the importance of CE-MRI markers of stroke risk over conventional measurements of luminal narrowing.
This is the first in vivo study demonstrating the combined roles of neovascularity and IPH in cerebrovascular ischemic events, and it was possible because of the ability of CE-MRI to reliably grade neovascularity by the enhancement of adventitia and to accurately detect IPH. Our results extend those of pathologic studies, which are incapable of analyzing adventitia because this is not generally included in an endarterectomy specimen.
Recognizing neovascularity as an important marker of plaque vulnerability has prompted the development of imaging approaches to its detection, with adventitial enhancement being the primary target. Staub et al23 used contrast-enhanced sonography and showed that adventitial enhancement in carotid plaque was associated with a history of cardiovascular disease. Romero et al24 retrospectively reviewed CT angiograms and showed that carotid wall enhancement (ie, depicted as adventitial enhancement) in patients with ≥70% stenosis was more common in symptomatic than in asymptomatic patients. These modalities support adventitial enhancement as a valid target, though they cannot provide the soft-tissue contrast offered by MR imaging to identify other plaque components, such as IPH. Kerwin et al10,12 demonstrated the feasibility of quantifying adventitial vasa vasorum by dynamic CE-MRI perfusion imaging. Our results indicate that the postcontrast BBMRI images commonly used for plaque characterization8,9 are also capable of categorizing neovascularity by adventitial enhancement. This highly reproducible technique can be easily implemented in clinical practice and offers immediate insight into adventitial vasa vasorum as a marker of neovascularity without requiring specialized sequences and more complex labor-intensive MR image postprocessing and data analysis.10,12
Although a full plaque analysis10 by CE-MRI could provide a comprehensive survey of neovascularity, the heterogeneous array of plaque components enhance to varying degrees; this feature makes neovascularity difficult to ascertain without measuring dynamic contrast uptake. Nevertheless, the adventitia serves as an appropriate target, given that neovessels are thought to arise here and progress into the intima,5,25 and the added value of a full plaque assessment remains to be shown.
As expected based on pathologic evidence that IPH stimulates plaque progression,5 we observed that IPH was more prevalent in larger plaques. Despite pathologic evidence that IPH develops from ruptured neovessels,4 we found that IPH presence and adventitial enhancement grade were independently associated with ipsilateral cerebrovascular events. Perhaps this could relate to pathologic studies that have shown that IPH can also originate from fissures or cracks in symptomatic coronary plaques26 that might be remote from the adventitia, or that aortic plaque neovascularization is associated with macrophage infiltration and thin-cap atheromas and correlates with rupture independent of IPH.27 Our findings are further supported by recent evidence that IPH presence and increased neovessel density in carotid specimens are independently associated with a risk of future cardiovascular events.28
Although stenosis is routinely used as a measure of stroke risk, its lack of association with events here is not surprising. The European Carotid Surgery Trial reported that 43.8% of the 3018 participants with symptomatic carotid disease had <30% stenosis.29 Furthermore, previous MR imaging reports have identified high-risk carotid plaques with IPH and fibrous cap rupture in arteries with low-grade (<50%) stenosis.1,30 The nature of asymptomatic referrals (ie, these patients are more likely to be identified when the narrowing is hemodynamic and results in a bruit) and the presence of bulb lesions (ie, no measurable stenosis by NASCET criteria despite luminal indentation) could influence the ability to detect a significant association between stenosis and symptoms. However, the plaque size (ie, mean or maximum wall thickness) was not different between symptomatic and asymptomatic patients.
Limitations include the following: 1) associations with prior events. Although associations with future events would be more credible, prior rupture does convey future risk. Inzitari et al31 showed that the risk of stroke in the territory of an asymptomatic carotid artery is substantially less than the risk in the territory of a symptomatic artery with a similar degree of narrowing. A prospective study of these features is now needed to validate their value as markers of future events. 2) We could not correlate MR images with endarterectomy specimens, when available, because surgical specimens lack adventitia. Finally, although gadolinium serves as an intravascular agent that can highlight the neovascular bed within adventitia, there may be extracellular extravasation on delayed images from underlying inflammation that contributes to this enhanced signal intensity. Further studies correlating the results of adventitial enhancement by MR imaging with that measured by using intravascular microspheres by sonography would help to elucidate the underlying mechanism.
Conclusions
High-resolution CE-MRI can stratify carotid plaque stability on the basis of neovascularity and IPH presence, both of which are independently associated with recent ischemic events. These features may provide insight into the risk for future cerebrovascular ischemic events not achievable by conventional stenosis measurements.
Acknowledgments
We acknowledge Sebastian Kelle, MD, and Saurabh Malhotra, MD, for their help in acquiring some of the MR images.
References
- Received April 22, 2011.
- Accepted after revision July 18, 2011.
- © 2012 by American Journal of Neuroradiology