Abstract
SUMMARY: The number of potential patients who are actually treated for acute ischemic stroke is disappointingly low, and effective treatments are making a minor impact on this major public health problem. Imaging is not regularly used to identify the ischemic penumbra, a key concept in stroke physiology, though it is capable of doing so in a clinically relevant manner. Evidence is accumulating that identification of the ischemic penumbra and making treatment decisions on the basis of its presence provide substantial benefit to patient outcomes. Moreover, the same studies suggest that an unexpectedly large proportion of patients are suitable for therapy well past the traditional time windows because of the existence of a substantial ischemic penumbra. Modern MR imaging and CT systems, now widely available, are capable of answering the most relevant physiologic questions in acute ischemic stroke. This capability presents new opportunities and responsibilities to neuroradiologists to make appropriate imaging readily available and to have the imaging data rapidly processed and interpreted. In this article, acute ischemic stroke therapy, including the role of imaging in current medical practice, is reviewed, and an evidence-based alternative to contemporary acute ischemic stroke therapy is suggested.
In the 1990s, the “Decade of the Brain,” exciting developments in the treatment of acute ischemic stroke and in neuroimaging promised a new era in which the third leading cause of death and the leading cause of severe disability would be brought under control. After a major trial published in the New England Journal of Medicine, tissue plasminogen activator (tPA) was approved by the United States Food and Drug Administration (FDA) for use as an intravenous agent in ischemic stroke.1 Concurrently, advances in neuroimaging, especially diffusion/perfusion MR imaging, demonstrated that the “Holy Grail” of stroke physiology, the ischemic penumbra, could be identified clinically.2 Hopes were enhanced by further developments in stroke therapy, such as intra-arterial chemical3, 4 and mechanical recanalization5 and the emergence of high-speed CT. Nevertheless, expectations for a rapid conquest of ischemic stroke proved to be premature. Indeed, it is estimated that only a small proportion of potential patients receive therapy.6–8 Moreover, advanced neuroimaging is rarely used in the routine evaluation of these patients.
So what happened? One potential reason for this state of affairs is that time of stroke onset has assumed a preeminent role, suppressing other factors, including the use of physiology to guide stroke therapy. This assumption may have had unintended, unexpected, and unfortunate results. The purpose of this review is to examine the current state of stroke therapy, including the major effect of time in its guidance, the evidence for neuroimaging to improve treatments through its visualization of relevant physiology, and how radiologists should facilitate neuroimaging to benefit the patient with stroke.
Current Acute Ischemic Stroke Treatments
Current FDA–approved treatments for acute ischemic stroke include intravenous fibrinolytics and endovascular approaches.2 Despite proved efficacy of some therapy, the number of patients treated for acute stroke remains disappointingly low.
Intravenous tPA.
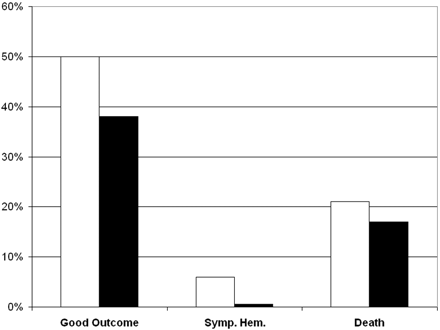
tPA is the only drug currently authorized by the FDA for acute ischemic stroke treatment. FDA approval was based on the results of the NINDS tPA Stroke Study.1 Three major measures from that trial are depicted in Fig 1. In the 624 patients studied, 50% of tPA–treated patients had excellent global outcomes (minimal or no deficit) compared with 38% of controls at 3 months after treatment. However, a higher rate of symptomatic hemorrhage in the tPA group (6.4% versus 0.6%) was observed. The mortality rate at 3 months was not significantly different between groups. On the basis of the NINDS trial and the previously reported experience, intravenous tPA was approved in the United States, and its use endorsed in consensus guidelines9, 10 for treatment of acute ischemic stroke. Further support for the use of tPA came from pooled data analysis from several trials,11 meta-analysis,12 phase IV studies,13 and studies based on routine clinical practice.7,14–23
Three outcome measures from the NINDS tPA trial.1 In a study population of 624 patients, recombinant tPA (white bar) or placebo (black bar) was administered intravenously in patients within 3 hours of stroke symptom onset and negative findings on head CT. Displayed here are percentages of patients who had good clinical outcomes at 3 months (modified Rankin Score of 0–2), symptomatic hemorrhage, and death. The long-term clinical benefit of tPA was significant (global odds ratio for a favorable outcome, 1.7; 95% confidence interval, 1.2–2.6). Compared with patients given placebo, patients treated with tPA were more likely to have minimal or no disability at 3 months on the assessment scales (50% versus 38%). Symptomatic intracerebral hemorrhage within 36 hours after the onset of stroke occurred in 6.4% of patients given tPA but in only 0.6% of patients given a placebo (P < .001). Mortality rate at 3 months was 17% in the tPA group and 21% in the placebo group (nonsignificant, P = .30).
Evidence-Based Recommendations for Use of tPA.
At the seventh American College of Chest Physicians Conference on Antithrombotic and Thrombolytic Therapy: Evidence-Based Guidelines, the following course of action was recommended24: For eligible patients, intravenous tPA should be administered intravenously with a dose of 0.9 mg/kg (maximum of 90 mg), with 10% of the total dose administered as an initial bolus and the remainder infused over 60 minutes, provided that treatment is initiated within 3 hours of clearly defined symptom onset. Evidence-based guidelines were also given for patients with extensive (greater than one third of the MCA territory) and clearly identifiable hypoattenuation on CT. The recommendation was against giving thrombolytic agents to patients with these criteria. Minor ischemic changes that are commonly present were not considered a contraindication to treatment.24
Low Rate of Use of Intravenous tPA.
Despite the many studies, FDA approval, and nearly a decade’s experience with its routine clinical use, tPA is underused. It has been reported that only about 1%–7% of potentially eligible patients in the United States actually receive this recommended therapy.6–8 There are several reasons, but the major obstacle is the inability to receive and treat the patient within the recommended 3-hour time window. Other issues include the fear of hemorrhage, inaccessible CT scans, and inadequate/uninformed personnel at many emergency departments. The disappointing result has been that intravenous tPA for the treatment of stroke has made only a minor impact on a major public health problem.
Intra-arterial Recanalization.
Multiple centers with active stroke services and with appropriately trained interventionalists have ongoing programs to treat acute stroke patients endovascularly. This approach is typically restricted to patients who have an occlusion of a major cerebral artery accessible by a microcatheter and are within 3–5 hours beyond the 3-hour-window restriction of intravenous tPA. Endovascular options include mechanical recanalization, chemical thrombolysis, or a combination of the two. Some centers follow intravenous tPA with an endovascular approach.
Mechanical Thrombolysis.
The Concentric Retriever (Concentric Medical, Mountain View, Calif) is the only device currently approved by the FDA for the endovascular treatment of stroke patients. The Mechanical Embolus Removal in Cerebral Ischemia (MERCI) Trial5 reported the use of this device in 141 patients with occlusion involving either the internal carotid artery (ICA), the M1 segment of the middle cerebral artery (MCA), or the basilar or vertebral arteries, within 8 hours of symptom onset. None of the patients were eligible for intravenous thrombolysis. Recanalization was achieved in 46% (69/151) of patients on intention-to-treat analysis and in 48% (68/141) of patients in whom the device was deployed. Of those patients, 22 had good functional outcomes measured at 90 days after treatment.5 The direct probing of the thrombus with the microguidewire is the most common mechanical approach to recanalization.25 A variety of mechanical approaches have been reported,26–33 including novel mechanical devices.34–36
Intra-arterial Thrombolysis (IAT).
The goal is to recanalize a vessel by direct infusion of a fibrinolytic agent into the clot causing the arterial occlusion. Several small series have been published that suggested the efficacy of this approach by using urokinase. In an analysis by Lisboa et al3 of 27 studies with 852 patients who received IAT and 100 control subjects, there were more favorable outcomes in the IAT than in the control group (41.5% versus 23%, P = .002), with a lower mortality rate for IAT (IAT, 27.2%; control group, 40%, P = .004). However, symptomatic intracerebral hemorrhage (ICH) was more frequent in the IAT group, compared with the control group (9.5% versus 3%, P = .046).3
In the most well-designed studies to date, the safety and efficacy of IAT in the anterior circulation was evaluated in 2 randomized multicenter placebo-controlled trials. In the Prolyse in Acute Cerebral Thromboembolism (PROACT) I and II trials, patients with proximal MCA (M1 or M2 segment) occlusions within 6 hours of symptom onset were treated with recombinant prourokinase (rpro-UK) or a placebo4, 37. In the PROACT II trial, 180 patients were enrolled.4 The primary clinical outcome, the proportion of patients with slight or no disability at 90 days (modified Rankin Score of ≤2), was achieved in 40% of the 121 patients in the rpro-UK treatment group compared with 25% of the 59 patients in the control group (P = .04). The recanalization rate was 66% for the rpro-UK group and 18% for the control group (P < .001). Symptomatic ICH within 24 hours occurred in 10% of rpro-UK patients and 2% of control patients (P = .06).38 The mortality rate was 25% for the rpro-UK group and 27% for the control group. Although the results were encouraging, the study did not result in FDA approval of rpro-UK.
The use of endovascular approaches is restricted to the recanalization of major cerebral arteries, most commonly the MCA. Major efforts are exerted in these cases of major artery occlusion because the outcomes are devastating if untreated and because of evidence that purely intravenous approaches are usually ineffective. These types of strokes constitute approximately 20% of the ischemic strokes that present to our institution. If this rate is similar across the United States, then it is estimated that the annual incidence of these strokes number well over 100 000. It is likely that no more than 1000 of these cases are treated endovascularly per year. Thus, the endovascular approach has had a minor impact on a major problem.
Imaging and Current Stroke Therapy.
The major determinants of the decision to proceed with a stroke treatment are the clinical presentation, the duration of stroke onset, and essentially negative findings on a noncontrast CT scan. Despite the increasing availability of physiologic evaluation of the brain by advanced CT and MR imaging techniques, they are not generally used to select patients for intravenous or endovascular therapy. Perhaps the major reason is that such methods can be costly with respect to time and “time is brain.” This situation is unfortunate because the identification of the “ischemic penumbra” could lead to more rational treatment decisions, yielding successful outcomes outside current time windows. However, this circumstance may change because of recent evidence suggesting treatments can be effectively administered beyond traditional time windows, when clinical neuroimaging can detect the ischemic penumbra.
The Ischemic Penumbra
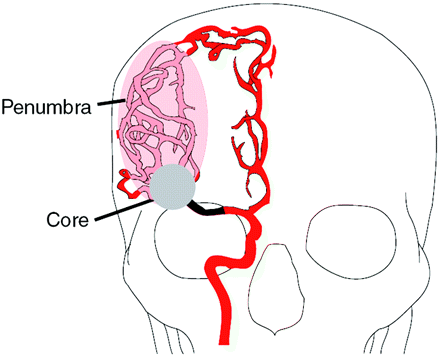
Ischemic stroke produces a severe reduction of blood flow to the brain, which leads to cell death by a variety of mechanisms including excitotoxicity, ionic imbalance, oxidative/nitrosative stress, inflammation, apoptosis, and peri-infract depolarization.39 Within areas of diminished blood flow, excitotoxic and necrotic cell death occurs within minutes. This is the “core” of the infarct, and tissue rapidly undergoes irreversible injury. However, cells in the peripheral zones are supported by collateral circulation. This peripheral region, termed the “ischemic penumbra,” contains tissue that may be salvaged with prompt institution of the appropriate therapy (Fig 2).40–43
The ischemic penumbra. Occlusion of a major cerebral artery such as the proximal right MCA, illustrated here, results in changes in the hemodynamics of the brain that vary from patient to patient. With occlusion, collateral vascular channels can provide blood flow to ischemic regions. Here, collateral circulation from the right anterior cerebral artery distribution to the right MCA territory is depicted. The perfusion within the vascular territory of the occluded artery varies with some areas receiving little blood flow, whereas other areas receive near-normal amounts of blood flow. This results in 2 regions: an infarction core that represents irreversibly injured brain and an ischemic penumbra that may be clinically symptomatic but can be rescued if blood flow is restored.
The concept of the ischemic penumbra was developed from studies using experimental animal models of ischemic stroke. Studies using nonhuman primates were among the most informative and most relevant to clinical stroke. Jones et al,44 nearly 25 years ago, reported observations using temporary and permanent occlusion of the MCA in rhesus macaques. These observations were robust and directly applicable to the human condition. Some of the original data are presented in Fig 3. Jones et al established that thresholds exist for cerebral ischemia, and these are related to cerebral blood flow (CBF) and the duration of reduced CBF. Clinical symptoms are observed with the reduction of CBF below a certain threshold, but tissue remained viable even if this reduced CBF persisted for several hours. However, below a lower threshold, cerebral infarction ensued at some point, depending on the precise CBF. At very low CBFs, cerebral infarction ensued within minutes; at higher levels of CBF (on the order of 15 mL/100 g per minute), 2 hours or more would be required before infarction occurred.
Blood flow and infarction. Data from awake monkeys in which an MCA was occluded.44 The open circles represent local blood flow in normal tissue, the open triangles are measurements in the periphery of the infarct, and the closed rectangles depict blood flow within the infarct. The dashed line approximates the infarction threshold; when the local CBF crosses this line, there is irreversible injury. Local CBF outside this line, but below normal levels (∼20–25 mL/100 g per minute), represents the ischemic penumbra.
Subsequent studies in humans using positron-emission tomography40, 45 confirmed the observations in animal models. In the clinical setting, it is generally not possible to determine the diminished CBF and its duration with precision. Different parts of the brain are likely to have different CBF as a result of compensatory blood flow via collateral channels. The physiology during an acute ischemic event is highly complex. Nevertheless, the complexity can be resolved by using modern CT and MR imaging methods. The core of the infarct may be estimated with a reasonably high probability. Moreover, though precise CBF values may be difficult to derive, a clinically useful estimate of the ischemic penumbra is also readily available.
MR Imaging and CT of the Ischemic Penumbra
Many, probably most, centers in the United States that treat patients with acute stroke have current CT and/or MR imaging scanners capable of physiologic imaging. Although current accepted practice requires only a noncontrast CT scan to initiate intravenous or IA thrombolytic therapy, the opportunity exists for improving treatment efficacy and greatly expanding the number patients treated through the use of physiologic imaging. There is strong evidence that the means are available for the reliable identification of the ischemic penumbra in a clinically relevant manner. Specifically, CT and/or MR neuroimaging are capable of identifying the regions of hypoperfusion and estimating areas of the brain that are irreversibly injured.
Imaging Abnormal Hemodynamics in Ischemic Stroke.
The most commonly used MR and CT perfusion approaches rapidly acquire a series of images from the same sections during a bolus infusion of intravenous contrast material. Postprocessing of the source images produces a series of maps that describe the cerebral hemodynamics. Most analytic methods describe the change in signal intensity as a function of time on a voxel-by-voxel basis. Time-to-peak (TTP) maps are simple to derive; they indicate the time required for the maximal signal intensity change within a voxel after the infusion of a contrast bolus. Measurement of the slope of signal intensity change is an approximation of CBF, whereas the full width of the signal-intensity-change curve at half of maximum value is an approximation of the contrast transit time. The area under the signal-intensity change–time curve is proportional to the cerebral blood volume (CBV). The deconvolution method requires a separately measured arterial input function, which allows computation of a residue function describing the fraction of a hypothetic instantaneous contrast bolus that would remain at each time after injection. The peak of the residue function is proportional to CBF. CBV is divided by CBF to yield the mean transit time. There are additional hemodynamic maps that can be derived.2
Perfusion imaging can be performed with high-speed MR imaging and CT systems that are commonly available. Perfusion imaging with MR imaging has been validated in animal models and in clinical stroke studies.46–56 Validation studies of perfusion imaging by CT have been conducted by a number of clinical investigations.38, 52,57–66 Postprocessing methods of perfusion data are similar for both MR imaging and CT. There have been investigations comparing the reliability of various perfusion maps. Although some studies have indicated that the deconvolution methods are superior,56 a recent evaluation comparing nondeconvolution and deconvolution methods performed by our group found that they are basically equivalent in identifying perfusion abnormalities that are clinically relevant.
Imaging the Infarct Core.
Of the available MR imaging and CT measurements, abnormalities detected on diffusion MR images are considered the most reliable estimate of the ischemic core—that is, tissue that is very likely to be irreversibly injured. The evidence supporting this finding is substantial. Diffusion-weighted MR images (DWI) are highly sensitive and specific in the detection of brain injury early in ischemic infarctions.49, 53,67–69 In significant cerebral ischemia, diffusion is only normal if the lesion is very small, such as in lacunar infarctions, if hypoperfusion is very low, or if it is very early in the course of ischemia. In the absence of therapy, ischemic diffusion abnormalities rarely reverse.55 With early reperfusion following thrombolysis, some reversal of diffusion abnormalities can be observed.70, 71 Close examination of such patients have revealed that areas that undergo reversal typically have very mild depression of the apparent diffusion coefficient, indicating that these areas have been subjected to a relatively mild reduction in blood flow or that it is very early in the course of ischemia.71 Taken together, the evidence indicates the signal intensity abnormality observed on diffusion MR imaging is the best current marker of the infarction core.51
The infarction core can also be estimated by certain CT methods. A visible hypoattenuation on the noncontrast CT scan rarely reverses. Early in ischemic stroke, the findings on noncontrast CT are commonly normal. In many of these cases, abnormalities seen on the CT angiography source images may be a marker of the core.52 Another reasonably reliable marker of the core is a CBV abnormality identified in CT perfusion data.59
Identifying the Ischemic Penumbra.
The difference between the ischemic core and the extent of the abnormally reduced perfusion constitutes, for practical clinical purposes, the “operational ischemic penumbra.”51 This is supported by evidence showing that in the absence of treatment, the final infarct size will grow larger than the core and may attain the size of the operational ischemic penumbra. The most well-evaluated method is diffusion/perfusion MR imaging. A large number of studies have been published over the past 10 years demonstrating that the identification of a “diffusion/perfusion mismatch” heralds the growth of the infarct core in the absence of therapy.46, 51,72–79 There have been a number of studies investigating which hemodynamic map best predicts tissue infarction in the absence of treatment. The evidence suggests that there may be several clinically adequate approaches. More important, there is growing evidence that in the absence of a diffusion/perfusion mismatch, therapy is likely to be futile.80, 81 There are less published data on the use of CT to define the ischemic penumbra. Several investigations have demonstrated that CT can give information similar to that provided by MR imaging59, 82 and that the ischemic penumbra identified by CT perfusion imaging predicts growth of infarct size in the absence of treatment.59, 83
Evidence Supporting Imaging for the Selection of Patients for Acute Ischemic Stroke Therapy
Two recently published articles demonstrate how the neuroimaging of the ischemic penumbra can successfully guide therapy. In one study, a new fibrinolytic agent was evaluated.80 In the other study, tPA was used; however, it was given outside the normal time window.81 In both studies, the decision to treat required the demonstration of an ischemic penumbra by using diffusion/perfusion MR imaging.
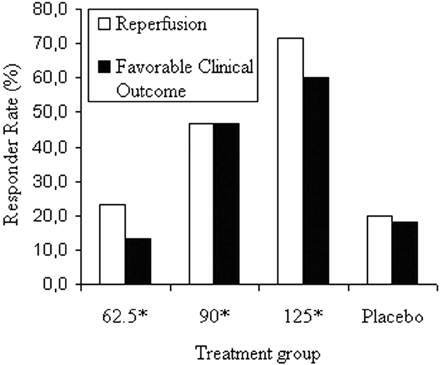
In the Desmoteplase in Acute Stroke (DIAS) Trial,80 a new fibrinolytic agent or placebo was administered intravenously 3–9 hours after the onset of symptoms. Additional requirements included the finding of a diffusion abnormality incorporating less than one third of an MCA territory (indicating a relatively small infarct core), a perfusion abnormality of greater than 2 cm, and a perfusion/diffusion mismatch of greater than 20%. More important, there were few restrictions as to the type of perfusion maps required for this determination. In most centers, the simplest perfusion estimates, typically TTP maps, were used. In the second part of this phase II study, a dose-escalation protocol was followed. At the optimal dose of 125 μg/kg of desmoteplase, 60% of patients had a good outcome, compared with 20% who received a placebo (P = .009). Figure 4 depicts some of the key findings from DIAS study. The strong statistical significance, despite a small number of subjects (n = 15 for desmoteplase and n = 11 for the placebo), indicates the power of using an effective treatment coupled with patient selection based on the demonstration of an ischemic penumbra.
Outcomes and reperfusion rates in the Desmoteplase in Acute Stroke (DIAS) trial.80 Data from the dose escalation part of this phase II trial are depicted, including reperfusion percentage (open bars) and good clinical outcomes (black bars). Dosage on the horizontal axis is in units of micrograms per kilogram. At the optimal dose (125 μg/kg), the reperfusion percentage (∼70%) and good outcome percentage (∼60%) were much superior to those of a placebo. Remarkably, strong statistical significance was achieved (P = .009), despite small numbers of patients (n = 15 for desmoteplase at the optimal rate and n = 11 for the placebo).
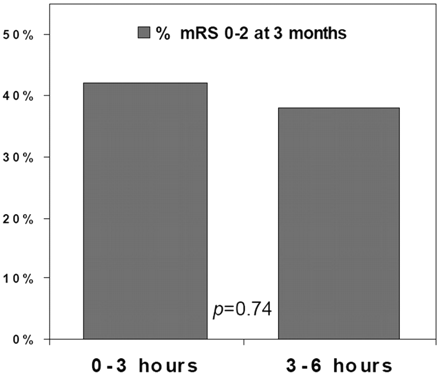
In a single-center study, Ribo et al81 reported the efficacy of tPA administered to eligible patients 3–6 hours after the onset of stroke symptoms. Patients presenting with an acute stroke syndrome were placed into 2 groups. Group A included all patients who arrived within 3 hours of stroke onset and met the standard criteria for the administration of intravenous tPA. These patients underwent the standard protocol, and only a noncontrast CT scan was obtained. Patients in group B were eligible to receive intravenous tPA 3–6 hours after stroke symptom onset if they met the MR imaging criteria, including a perfusion/diffusion mismatch of 50% or greater. Surprisingly, nearly 75% of the patients that arrived within the 3- to 6-hour time limit met the diffusion/perfusion criteria. The primary end point was clinical outcome at 3 months (modified Rankin scale of 0–3). The outcomes for groups A and B were similar, with approximately 45% of patients having a good clinical outcome (Fig 5).
Outcomes in extended time window for tPA trial.81 Patients within 0–3 hours from symptom onset (group A, n = 79) were treated according to standard CT criteria. Treatment within 3–6 hours (n = 43) was decided according to protocol criteria that included a diffusion- and perfusion-weighted image–generated TTP map; criteria for tPA treatment were defined as a diffusion/perfusion mismatch >50%. Clinical outcomes for the patients in the 2 groups were not significantly different. Interestingly, more than 75% of the 56 patients considered for inclusion into the 3- to 6-hour group B had a diffusion/perfusion mismatch >50%.
These 2 studies provide several important lessons. First, the time window after stroke onset may be quite wide for many patients. Second, these studies validate the use of clinical imaging to define the ischemic penumbra. Finally, these studies show that the ischemic penumbra can be used to guide therapy in a clinically relevant manner. In considering the data from these studies, we believe that the impressive statistical outcomes of these studies may well be due to a (beneficial) selection bias: the exclusion of patients with an absence of an ischemic penumbra. This begs the question of what percentage of patients presenting within the first few hours after stroke onset have a substantial penumbra. The percentage may be large, as suggested by the study by Ribo et al.81
CT Versus MR Imaging in Acute Ischemic Stroke
CT and MR imaging are widely available and provide information on the state of the brain parenchyma, the vessels, and brain tissue perfusion in patients with acute stroke. Because many institutions have the capability to perform either or both imaging techniques, the question is which one to use. Although the question is practical, the answer is problematic and depends on many factors, including the state of the patient, the time since stroke onset, the logistical constraints of using one or the other technique, and the capability of a facility to perform advanced therapy, such as intra-arterial thrombolysis.
In clarifying this issue, one must consider the salient clinical questions requiring immediate determinations. There are 4 key observations: (1) Is there a hemorrhage? (2) Is a large vessel occluded? (3) What part of the brain is irreversibly injured? and (4) Is there a penumbra? Both CT and MR imaging provide information to answer each of these questions. However, the techniques are not equal. We shall consider detection/delineation of each finding while assuming the availability of state-of-the-art CT and MR imaging.
(1) Is There a Hemorrhage?
CT is superior. Both techniques can detect clinically significant parenchymal hemorrhages; however CT remains superior in detecting acute subarachnoid hemorrhage,84 which is an important consideration in the acute stroke patient. MR imaging is less sensitive because the high oxygen levels in CSF result in erythrocytes maintaining near-normal levels of oxyhemoglobin. Also fluid-attenuated inversion recovery images are less reliable in assessing early hemorrhage in the basal cisterns and posterior fossa.
(2) Is a Large Vessel Occluded?
CTA is superior. The question of whether the ICA, MCA M1 or M2 branch, or the basilar artery is occluded is extremely important, especially if the capability exists for intra-arterial intervention. Under optimal conditions, MR angiography (MRA) is equivalent to CTA, but conditions are seldom optimal when dealing with the acute stroke patient. The longer time required to acquire an MRA is an important consideration, but of greater importance is the vulnerability of MRA to motion artifact that commonly results in an MRA of poor quality. CT angiography (CTA) more reliably produces high-quality angiographic information of both the head and neck vessels in less than 2 minutes.85
(3) What Part of the Brain is Irreversibly Injured?
Diffusion MR imaging is superior. CTA source images and CBV measures provide an estimate of irreversible injury, but they are less sensitive than DWI.52
(4) Is There a Clinically Relevant Ischemic Penumbra?
Perfusion MR imaging is superior. Both methods provide information of similar quality. However, MR imaging systems can evaluate a larger proportion of the brain.86
The answer to each question is based on review of the literature and the practical everyday clinical experience of dealing with stroke patients who come to our hospital.2 So what do we use at the Massachusetts General Hospital? The answer is both techniques whenever possible. We perform both in most acute stroke cases because we are fortunate to have in our emergency department state-of-the-art CT and MR imaging that are located close to each other. The studies are also rapid, with CT/CTA/CT perfusion requiring 10 minutes or less and the MR imaging being restricted to diffusion, perfusion, and perhaps 1 additional sequence taking no more than 10 minutes of imaging time. An example of our approach is shown in Fig 6. Many institutions will have CT and MR imaging in different locations, raising logistic issues. The optimal solution obviously varies from institution to institution and from patient to patient. Time may be required to move a patient to the MR imaging scanner, which may be located in another part of the hospital. Nevertheless, it is becoming clear that for many, possibly most patients, the ischemic penumbra may be stable for several hours and the value of visualizing the physiology provides the information necessary for safe and effective treatment.
Using imaging-derived physiologic information to guide therapy. The patient is a 38-year-old man who was found with a severe left hemiparesis. The precise time of stroke onset was unknown. The noncontrast CT scan was remarkable for a hyperattenuated focus in the region of the right MCA (not shown). A, CT angiography demonstrates occlusion of the right MCA (arrow), but with reconstitution of distal branches likely due to collateral circulation. B, Diffusion MR imaging shows abnormal diffusion in the distribution of the right lenticulostriate arteries, but with sparing of the remainder of the MCA territory, including the cerebral cortex. C, Perfusion MR imaging displays abnormalities involving the entire right MCA territory. The time-to-minimum-perfusion map at the same level as the DWI image demonstrates a perfusion deficit that is much larger than the DWI abnormality. Because of the large diffusion/perfusion mismatch and the likely poor long-term outcome in the absence of treatment in this man with a young family, the decision was made to proceed to intra-arterial thrombolysis. D, Right common carotid angiogram demonstrates a cutoff of the right MCA, corresponding to the previously obtained CTA (arrow). E, After manipulation with a microguidewire and infusion of urokinase, right MCA flow was established (arrow). F, A follow-up head CT scan shows infarction in the region of the right corona radiata and a small portion of cerebral cortex. The patient made an excellent recovery with minor neurologic deficits at discharge.
Radiology Opportunities, Challenges, and Solutions
The move toward rational imaging-based therapy for acute ischemic stroke based on objective physiologic criteria creates new responsibilities for radiologists. The sheer number of individuals who have an acute ischemic cerebral event, the need to assess brain physiology, and the widespread availability of effective therapies place new demands on radiology departments. Foremost is the need for readily available current CT and/or MR imaging scanners. The wide distribution of multidetector CT and echo planar–capable MR imaging throughout the country suggests that this requirement has been largely met or will be in the near future. Two additional factors include the processing of the large volume of data that these scanners generate and the availability of radiologists to interpret these studies.
Image Data Processing.
The data generated by modern scanners in patients being evaluated for ischemic stroke are enormous. Hundreds of images are generated by CT angiograms and perfusion data. The only way to rapidly and accurately evaluate these data are through postprocessing of the data. To be useful, the postprocessing of imaging data must occur immediately—that is, the technologist at the CT or MR imaging console must be able, in short order, to generate views of CT angiograms, diffusion images, and perfusion images. At our institution, we are fortunate to have a laboratory dedicated to the 3D processing of images, but the time delay required for the transfer of data and subsequent processing of the data was too long for it to be practical in ischemic stroke (unpublished data). We also found, unfortunately, that viewing the source images can often be misleading. We now use a relatively simple solution. We ask the technologist to reconstruct maximal intensity projection images from 3-cm-thick slabs from the CTA dataset in 5-mm increments. We also require that these thick-slab MIP reconstructions be performed in 3 orthogonal planes. These can be created by the technologist at the console in a short period of time, 5 minutes or less (Fig 6A). In our experience, these have been highly effective in clearly delineating occlusions of the basilar artery, ICA, and proximal MCA. This type of processing at the console is available in most modern scanners that are capable of acquiring CT angiographic data.
The generation of perfusion maps is another challenge. Here various vendors have developed programs that are relatively easy to run by the technologist at the scanner. This has been true for MR imaging for some time and is now occurring for CT. One of the rewarding lessons from DIAS is that you do not need the most sophisticated perfusion analysis to provide the necessary crucial information. Simple algorithms mapping TTP contrast delivery provide the essential information that is required for making crucial decisions.
Radiology Interpretation 24/7.
The last major challenge is the availability of the radiologist to interpret these studies 24/7. Here, the rapid advances in information technology provide potential solutions. It is impractical to have radiologists travel to the emergency department for each potential patient with ischemic stroke. However, now it is possible to rapidly deliver high-quality image data to radiologists wherever they may be. The most important factor is the widespread availability of high-speed internet access. This, plus the development of excellent Web-based PACS browsers, potentially resolves the problem of radiologist availability with a relatively modest investment of funds.
Conclusions
Advances in neuroimaging have made possible the reliable visualization of the relevant anatomy and physiology in acute ischemic stroke, most notably the ischemic penumbra. Additionally, new and standard treatments have been shown to be effective outside the traditional time windows when the penumbra is imaged. Implementing these approaches in a large patient population places new responsibilities and opportunities on radiologists, including the need to make modern CT and MR imaging readily available, along with the rapid postprocessing of image data and the timely review of those images. Fortunately, practical solutions to all these issues are now available, and with them, it is reasonable to imagine a future in which physiology-based rational imaging-guided ischemic stroke therapy becomes the clinical standard to the great benefit of our patients.
Footnotes
This work was supported in part by the National Institute for Neurological Disorders and Stroke grant NS50041 to R.G.G.
References
- Copyright © American Society of Neuroradiology