Graphical Abstract
Abstract
BACKGROUND AND PURPOSE: Early identification of malignant cerebral edema (MCE) in patients with acute ischemic stroke is crucial for timely interventions. We aimed to identify regions critically associated with MCE using the ASPECTS to evaluate the association between location-specific net water uptake (NWU) and MCE.
MATERIALS AND METHODS: This multicenter, retrospective cohort study included patients with acute ischemic stroke following large anterior circulation occlusion. The ASPECTS was determined by RAPID ASPECTS software. ASPECTS-NWU and Region-NWU were calculated automatically by comparing the Hounsfield unit values in the ischemic and contralateral regions. Critical ASPECTS MCE regions and Region-NWU were evaluated by multivariate logistic regression and the areas under the receiver operating characteristic curves (AUCs).
RESULTS: The study included 513 patients. Multivariate analysis showed that the ASPECTS insula (OR = 2.49; 95% CI, 1.44–4.31) and M5 (OR = 1.59; 95% CI, 1.11–3.41) regions were significantly associated with MCE. After adjustment, only the insula (OR = 2.34; 95% CI, 1.23–4.45) was independently associated with MCE. Univariable receiver operating characteristic curve analysis found AUCs for Insula-NWU (AUC, 0.70; 95% CI, 0.65–0.76) and ASPECTS-NWU (AUC, 0.64; 95% CI, 0.58–0.70). The Insula-NWU had better diagnostic power than ASPECTS-NWU (DeLong test; P = .01). A multivariate regression model that combined the NIHSS, ASPECTS, insula involvement, and Insula-NWU had good discriminatory power (AUC = 0.80; 95% CI, 0.74–0.86) and better diagnostic power than Insula-NWU (DeLong test; P < .01).
CONCLUSIONS: The insula region is critical for MCE, and Insula-NWU has better prediction efficacy than ASPECTS-NWU. This method does not rely on advanced imaging, facilitating rapid assessment in emergencies.
ABBREVIATIONS:
- AUC
- area under the receiver operating characteristic curve
- HU
- Hounsfield unit
- MCE
- malignant cerebral edema
- NWU
- net water uptake
- ROC
- receiver operating characteristic curve
SUMMARY
PREVIOUS LITERATURE:
NWU, which quantifies the low attenuation degree on NCCT, could be replaced by the automatically measured ASPECTS-NWU to predict the development of malignant cerebral edema in ischemic stroke after acute large-vessel occlusion independent of CTP.
KEY FINDINGS:
The insula region in ASPECTS is a strategically located region for predicting MCE. The Insula-NWU predicted MCE better than ASPECTS-NWU. Combining the NIHSS, ASPECTS, insula involvement, and Insula-NWU had a good predictive effect with an AUC of 0.80.
KNOWLEDGE ADVANCEMENT:
The Insula-NWU broadens the usability of the automatic ASPECTS software, helps simplify the NWU measurement, and could be an important indicator in future MCE prediction models.
Malignant cerebral edema (MCE), which often occurs after an occlusion of large vessels in the anterior circulation, is a life-threatening complication. It has a prevalence of 7.5%–25.9%,1⇓-3 with death due to tissue displacement and brain herniation in nearly 80% of patients.4 Early prediction of MCE in patients who could benefit from decompressive craniotomy might reduce mortality.4 Results from animal studies have shown a negative correlation between x-ray attenuation and hemispheric water content. Each 1% increase in tissue water content was associated with a decrease of 1.8 Hounsfield units.5 Consequently, the degree of low attenuation, as quantified by NCCT, is indicative of brain tissue water content, expressed as net water uptake (NWU). This is an increasingly used parameter to predict MCE in stroke research.6
Standardized NWU measurements require the definition of the infarct core using CTP, co-alignment of the infarct core region with the corresponding native CT, and measurement of the CT attenuation at the infarct core region and the contralateral brain tissue.6 The main drawback of CTP-based NWU measurements is the time-consuming steps of image segmentation and matching, the use of which is limited to patients who have undergone CTP scans.
The ASPECTS is a semiquantitative score used to identify the extent and distribution of early ischemic changes.7 The ASPECTS is divided into 10 regions based on the MCA blood supply with topologic location information. Studies have confirmed that the ASPECTS regions of infarcts at specific locations contribute differently to functional prognosis,8 but the relationship with the topologic location of MCE has been rarely investigated. For each percentage increase in water content, the absolute CT attenuation varies in the white and gray matter of the brain, reflecting that different regions of brain tissue might contribute differently to the development of MCE.9 It follows that the ASPECTS and NWU reflect 2 CT scan aspects of lesion progression following stroke. Whereas the ASPECTS is used to estimate the extent of the lesion, NWU can be interpreted as an indicator of the “depth of ischemia” per unit volume. The assessment of both is influenced by the location.10 Automatic ASPECTS software that measures the mean CT value of the defective (ischemic) region could calculate the ASPECTS-NWU, which could then be used to predict the lesion age11 and MCE.12 While the 10 ASPECTS regions range in importance and size from the caudate nucleus to the M1–M6 regions, the ASPECTS-NWU calculations do not consider the relationship between the location and MCE.
We hypothesized that the ASPECTS regions of infarcts at specific locations contribute differently to MCE and that quantified location-specific NWU could contribute to the predictive power of MCE. This study aimed to do the following: 1) identify ASPECTS regions independently associated with MCE, 2) identify the relationship between location-specific NWU and MCE, and 3) determine whether location-specific NWU could differentiate MCE better than ASPECTS-NWU.
MATERIALS AND METHODS
This study was approved by the local ethics committees of all participating institutions. The requirement for informed consent was waived by these committees. This study followed the methodology recommended in the Standards for Reporting Diagnostic Accuracy Studies (STARD) guidelines.
Patients
This retrospective, observational cohort study assessed consecutive patients who presented with acute ischemic stroke due to occlusion of a large vessel in the anterior circulation and received reperfusion therapy between June 2021 and June 2023 at 3 centers. Inclusion criteria were as follows: 1) 18 years of age or older; 2) NCCT images obtained within 12 hours of stroke onset; 3) occlusion of a large vessel in the anterior circulation (ICA or the M1 or M2 segment of the MCA) confirmed by CTA or MRA; and 4) treatment with reperfusion therapy (endovascular thrombectomy and IV thrombolysis). Exclusion criteria included baseline NCCT showing intracranial hemorrhage or intracranial tumor, additional infarction in the posterior circulation or anterior cerebral artery region, severe artifacts or incomplete images, failure of processing by the ASPECTS software, and lack of follow-up images to assess the MCE.
We retrieved baseline variables from the electronic medical records, including demographic characteristics, vascular risk factors, medical history, laboratory and imaging data, the NIHSS on admission, and time from stroke onset to CT.
The primary outcome was MCE, defined by the presence of a large occupying infarct of the MCA causing compression of the ventricles or a midline shift of ≥ 5 mm on the CT on days 3–5 of follow-up, consciousness level of ≥1 on item 1a on the NIHSS, and no other causes of neurologic deterioration. The midline was determined by drawing a straight line between the anterior and posterior portions of the cerebral falx. The midline offset was quantified by drawing a second parallel line at the position where the deviation from the midline was the greatest.13
Image Acquisition
All NCCT images from the 3 centers were in a DICOM format and generated by 1 of 4 multivector CT scanners (United Imaging uCT510, UIH; Somatom Definition, Siemens Healthineers; Sensation 64, Siemens Healthineers; Brilliance iCT, Philips Healthcare). The included NCCT images were acquired with a power of 120–40 kV, 170–380 mA, 2-second scan time, and 5-mm slice thickness.
Image Analysis
The ASPECTS was calculated using an automatic software tool (RAPID ASPECTS, Version 4.9; iSchemaView). The 10 regions evaluated for the ASPECTS were classified into subcortical (caudate [C], lentiform [L], internal capsule [IC], and insula [I]) and superficial cortical (M1–M6) regions. First, thick-slice NCCT images (5 mm) in DICOM format were imported for preprocessing, which included removal of the skull base and cranial and CSF spaces and tilt correction of the images. A standardized atlas was then applied to create individualized grids corresponding to the 10 ASPECTS regions on each hemisphere, and Hounsfield unit values were calculated for the 20 regions in the right and left brain hemispheres.
Each region was then classified as having normal or abnormal findings using a machine learning–based algorithm, and the most likely affected hemisphere was determined. Finally, an ASPECTS output map was generated, marking the affected region in red and reporting the ASPECTS.
On the basis of the regional Hounsfield unit (HU) obtained by the RAPID ASPECTS software, we calculated the mean HU values for the ischemic (HUischemic) and corresponding contralateral (HUnormal) regions and determined the NWU value for each according to the Equation. The ASPECTS-NWU was calculated using the average HU values for the overall ischemic and corresponding contralateral regions based on the ischemic region obtained from the RAPID ASPECTS software (Fig 1).11 Region-NWU was calculated using the HU values for individual ischemic and contralateral ASPECTS regions (Fig 1).

Measurements of ASPECTS-NWU and Region-NWU by RAPID ASPECTS software. A, The RAPID software shows an ASPECTS score of 6. The ischemic regions were the lentiform, insula, M3, and M6. Each region had a corresponding CT value. B, A 48-hour follow-up CT scan shows MCE in this patient. C, Calculations of the ASPECTS-NWU and Region-NWU according to the Equation. HUischemic was 30.38 (the mean HU of the 4 ischemic ASPECTS regions [lentiform, insula, M3, and M6]). HUnormal was 32.18 (the mean HU of the respective normal ipsilateral ASPECTS regions). Region-HUinsula was 28.3. Region-HUnormal was 30.2.
Statistical Analysis
Data were analyzed using statistical packages in R (R Foundation; http://www.r-project.org; Version 3.4.3) and Empower-Stats (http://www.empowerstats.com, X&Y Solutions). Continuous variables with a normal distribution are presented as means, while categoric variables are reported as No. (%). Baseline characteristics and stroke information in the MCE and no-MCE groups were compared using the χ2 test for categoric variables and the 1-way ANOVA and Kruskal-Wallis tests for continuous variables with normal and skewed distributions, respectively. Multivariate logistic regression analysis was used to assess the association between the ASPECTS regions and MCE. The NWU was calculated separately for individual ASPECTS regions and the entire ASPECTS ischemic region (ASPECTS-NWU). We report ORs with 95% CIs. Smoothed spline plots were created to graphically depict the link between the variables and the MCE. Receiver operating characteristic curve (ROC) analysis was used to assess the diagnostic performance and sensitivity and specificity metrics of variables such as Region-NWU, ASPECTS-NWU, and baseline NIHSS scores in predicting MCE. We generated the 95% CIs for the areas under the receiver operating characteristic curve (AUCs) using the exact method, also known as the DeLong method. The optimal cut-point is determined by the threshold that maximizes the Youden index, effectively balancing sensitivity and specificity. Statistical methods for model comparison were performed by the DeLong method, with P values corrected for Bonferroni correction and statistically significant at .025.
RESULTS
Patient Characteristics
The study flow chart is presented in Fig 2. Among the 607 consecutively included patients with acute ischemic stroke due to acute large-vessel occlusion in the anterior circulation, 94 were considered ineligible for the study: 10 with cerebral hemorrhage on baseline NCCT scans; 2 with a brain tumor; 39 with a vertebral basilar, posterior cerebral, or anterior cerebral artery regional stroke; 15 with severe artifacts; and 28 with software analysis failure. Finally, 513 patients (314 men; mean age, 67.4 [SD, 12.3] years) were included in the study. The baseline clinical characteristics of the patients are shown in the Table.
Flow chart of patient selection for the study.
| Variable | All | MCE– | MCE+ | P Value |
|---|---|---|---|---|
| Age (mean) (yr) | 67.43 (SD 12.33) | 67.10 (SD 12.55) | 68.54 (SD 11.55) | .27 |
| Male sex, No. (%) | 314 (61%) | 241 (61%) | 73 (63%) | .67 |
| NIHSS score at baseline (mean) | 14.88 (SD 6.57) | 13.94 (SD 6.53) | 18.12 (SD 5.63) | <.01 |
| Time from stroke onset to CT (mean) (h) | 4.60 (SD 4.23) | 4.63 (SD 4.47) | 4.49 (SD 3.31) | .77 |
| ASPECTS (mean) | 6.11 (SD 2.83) | 6.58 (SD 2.57) | 4.52 (SD 3.09) | <.01 |
| ASPECTS-NWU (mean) | 5.87 (SD 3.87) | 5.49 (SD 3.79) | 7.20 (SD 3.85) | <.1 |
| Blood glucose (mmol/L) (mean) | 7.6 (SD 2.8) | 7.44 (SD 2.77) | 8.20 (SD 3.04) | .01 |
| Risk factors | ||||
| Diabetes mellitus, No. (%) | 125 (24%) | 100 (25%) | 25 (22%) | .41 |
| Hypertension, No. (%) | 329 (64%) | 252 (64%) | 77 (66%) | .59 |
| Previous ischemic stroke or TIA, No. (%) | 101 (20%) | 76 (19%) | 25 (22%) | .57 |
| Atrial fibrillation, No. (%) | 167 (33%) | 126 (32%) | 41 (35%) | .47 |
| Coronary artery disease, No. (%) | 67 (13%) | 50 (13%) | 17 (15%) | .56 |
| Occlusion site | .01 | |||
| ICA, No. (%) | 158 (38%) | 109 (35%) | 49 (50%) | |
| MCA-M1 segment, No. (%) | 231 (56%) | 184 (58%) | 47 (48%) | |
| MCA-M2 segment, No. (%) | 24 (6%) | 22 (7%) | 2 (2%) | |
| ASPECTS region, No. (%) | ||||
| M1 | 191 (37%) | 126 (32%) | 65 (56%) | <.01 |
| M2 | 182 (35%) | 119 (30%) | 63 (54%) | <.01 |
| M3 | 141 (27%) | 90 (23%) | 51 (44%) | <.01 |
| M4 | 140 (27%) | 94 (24%) | 46 (40%) | <.01 |
| M5 | 172 (34%) | 108 (27%) | 64 (55%) | <.01 |
| M6 | 135 (26%) | 87 (22%) | 48 (41%) | <.01 |
| Insula | 285 (56%) | 195 (49%) | 90 (78%) | <.01 |
| Lentiform nucleus | 248 (48%) | 176 (44%) | 72 (62%) | <.01 |
| Internal capsule | 231 (45%) | 164 (41%) | 67 (58%) | .02 |
| Caudate nucleus | 264 (51%) | 194 (49%) | 70 (60%) | .03 |
Clinical and imaging characteristics of the patient population
MCE was confirmed in follow-up CT in 116 (22.6%) patients. A comparison of data between patients with and without MCE is shown in the Table. Patients with MCE had higher NIHSS scores at admission (mean, 18.12 versus 13.94; P < .01), ASPECTS-NWU values (mean, 7.20 versus 5.49; P < .01), and blood glucose (mean, 8.20 versus 7.44 mmol/L; P = .01), and lower ASPECTS (mean, 4.52 versus 6.58; P < .01). Differences in all ASPECTS regions were statistically significant.
Primary Analysis: Analyses of the Association between the Region and MCE
Multivariate logistic regression analysis showed that the regions significantly associated with MCE were M5 (OR, 1.95; 95% CI, 1.11–3.41; P = .02) and the insula (OR, 2.49; 95% CI, 1.44–4.31; P = .01). The association with the remaining 8 regions (M1, M2, M3, M4, M6, lentiform nucleus, internal capsule, and caudate) did not reach statistical significance (Supplemental Data). After we adjusted for sex, age, NIHSS, and ASPECTS, the analysis showed that the insula region remained independently correlated with MCE (OR, 2.34; 95% CI, 1.23–4.45; P = .01), but the association with the M5 region was statistically insignificant (OR, 1.54; 95% CI, 0.80–2.97; Supplemental Data).
Secondary Analysis: Association between the Region-NWU and MCE
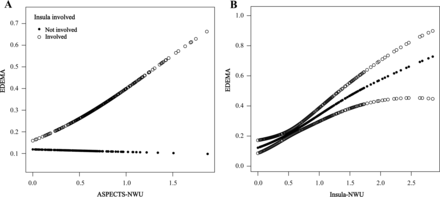
Figure 3A shows the relationship between ASPECTS-NWU and the MCE risk, stratified by insula involvement. When infarcts involved the insula, the ASPECTS-NWU increased and the risk of MCE was elevated, whereas if infarcts did not involve the insula, this association was absent. Figure 3B shows the association of insula-NWU with MCE.
The smoothing spline plots show the relationship between NWU and MCE. A, The relationship between ASPECTS-NWU and MCE risk, stratified by insula involvement. When infarcts involved the insula (hollow circles), ASPECTS-NWU and the risk of MCE increased, whereas when the infarcts did not involve the insula (black circles), this association was absent. B, The association between the Insula-NWU and MCE (black circle dotted line). The area between the 2 hollow circle lines is the 95% CI.
Because multivariate logistic regression analysis found that the insula and M5 regions were independently associated with MCE, they were combined to calculate the Insula+M5-NWU. Univariable ROC analysis found AUCs for Insula-NWU (AUC, 0.70; 95% CI, 0.65–0.76), Insula + M5-NWU (AUC, 0.71; 95% CI, 0.65–0.76), ASPECTS-NWU (AUC, 0.64; 95% CI, 0.58–0.70), and NIHSS (AUC, 0.70; 95% CI, 0.65–0.75). The diagnostic power of Insula-NWU was statistically better than that of ASPECTS-NWU (DeLong test; P = .01) (Fig 4). The optimal cutoff for Insula-NWU to classify MCE, the NWU value with the highest Youden index, was 5.75 (sensitivity 68.10%; specificity 66.50%). A model developed with NIHSS, ASPECTS, insula involvement, and Insula-NWU showed good discriminatory power with an AUC of 0.80 (95% CI, 0.74–0.86), sensitivity of 67.61%, and specificity of 80.08%. The diagnostic power of the model was statistically better than that of Insula-NWU (DeLong test; P < .01) (Fig 4).
ROC analysis resulted in AUCs of 0.64 and 0.70 for ASPECTS-NWU and Insula-NWU, respectively. The multivariate regression model combining NIHSS, ASPECTS, insula involvement, and Insula-NWU resulted in an AUC of 0.80. The diagnostic power of Insula-NWU is superior to that of ASPECTS-NWU by the DeLong test (P = .01), and the diagnostic power of the model is superior to that of Insula-NWU (P < .01).
DISCUSSION
This study aimed to identify the strategic locations associated with MCE and the efficacy of Region-NWU in predicting MCE in patients with acute ischemic stroke due to large-vessel occlusion in the anterior circulation. Our main finding was that the insula and M5 ASPECTS regions, especially the insula, were independently associated with MCE. Furthermore, Region-NWU was a more valid and simple predictor of MCE than the ASPECTS-NWU. Simplified NWU measurements could be made automatically with ASPECTS software, eliminating the need for advanced imaging and complex postprocessing and making it attractive for future applications.
The present study found that the insula was strongly associated with the development of MCE. The ASPECTS regions of infarcts at specific locations have been shown to contribute differently to functional prognosis. A recent meta-analysis found that infarcts were unevenly distributed across the ASPECTS regions and might be unevenly weighted in predicting the prognosis of patients with acute ischemic stroke, with infarcts in the M6 region being the strongest predictor.8 A study of the relationship between specific ASPECTS regions and good functional outcomes showed that the insula (OR, 0.56; 95% CI, 0.42–0.75) and M5 (OR, 0.53; 95% CI, 0.29–0.97) regions were negatively correlated with good functional outcomes and had the strongest effect.14
However, there are only a few studies on important positional features associated with MCE. A study based on voxel-based lesion-symptom mapping found that the lesion distribution in the temporal and frontal lobes was mildly-to-moderately predictive of the need for a decompressive craniectomy.15 However, the small sample of that study and the cumbersome postprocessing required when using the voxel-based lesion-symptom mapping approach make it difficult to apply to individual patients. Another study used the ASPECTS on follow-up CT, combining M2, M3, and the insula as the middle fossa. For patients without mechanical thrombectomy, the middle cranial fossa (OR, 2.57; 95% CI, 1.12–6.25) was independently associated with potentially lethal malignant edema.3 Our study showed that the insular region had good clinical interpretability of MCE. The insula is adjacent to the frontal/temporal opercula at the brain surface and medial to the Sylvian fissure and is directly supplied by the proximal portion of the 2 main MCA M2 branches after they branch at a right angle from the mainstem (M1), a topographic feature that predisposes them to embolisms, especially cardiac emboli, resulting in occlusion of the region.16
It has been reported that insula involvement might indicate a higher risk of conversion of salvageable penumbra to irreversibly damaged tissue,17 which is associated with a poorer clinical prognosis. In addition, co-involvement of the insula and M5 regions might have a more significant mass effect on the midline structure. Moreover, insula dysfunction might be directly or indirectly associated with stroke complications such as increased permeability of the BBB, hospital-acquired pneumonia, cardiac arrhythmias, and hyperglycaemia.18,19 The present study provides further evidence of the importance of insula infarction for the development of MCE.
Another important point was that the present study further combined the critical regions with NWU to reflect the relationship between the water content of the brain tissue in these regions and the occurrence of MCE. Broocks et al20 showed that it was possible to quantify NWU in infarcted lesions by measuring relative CT attenuation. They proposed NWU as an important alternative imaging biomarker for MCE in anterior circulation stroke. A study in patients with posterior circulation stroke similarly demonstrated a high discriminatory power for the occurrence of MCE when the NWU was >14.9% (AUC of 0.94).21 However, the standard procedure for measuring NWU in the above studies included CTP to ensure a precise definition of the core lesions used for attenuation measurements, limiting the clinical application of NWU. The ASPECTS-NWU, automatically measured by ASPECTS scoring software, is independent of CTP and could be used as an alternative measurement approach.11,12 However, it does not consider differences in importance among the ASPECTS regions and the varying degrees of water uptake among brain tissues.
The strength of this study is that it considers a strategically important region and combines NWU with it to yield the Insula-NWU with significantly better efficacy than ASPECTS-NWU in predicting MCE, further simplifying the assessment process. The combination of admission NIHSS, ASPECTS, insula involvement, and Insula-NWU resulted in a predictive AUC of >0.8. Therefore, Insula-NWU could be an important metric for subsequent predictive models. Moreover, it broadens the usability of the automatic ASPECTS software and helps simplify NWU measurements.
Our study had some limitations. First, it focused on the brain region significantly associated with MCE and the NWU in this region; however, the clinical prognosis of the patients was not analyzed. Related work will be performed in subsequent studies. Second, the NWU obtained on the basis of automatic ASPECTS software or CTP differs in measurement and cutoff values when predicting MCE, and this study could not compare the NWU values measured by the 2 methods. Third, the model in this study obtained the prediction results of MCE through multivariate logistic regression analysis, and it is possible that the results of the model were overly optimistic due to the lack of cross-validation.
CONCLUSIONS
The occurrence of MCE after acute ischemic stroke due to acute large-vessel occlusion is location-specific. The insula, a critical location, was combined with NWU to obtain a quantitative variable that could predict the occurrence of MCE. This approach does not rely on advanced imaging modalities, simplifying the MCE assessment and facilitating rapid assessment in emergency situations.
Footnotes
XiaoQing Cheng and Bing Tian contributed equally to this work.
This research was funded by the National Natural Scientific Foundation of China (82271983).
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received August 14, 2024.
- Accepted after revision December 11, 2024.
- © 2025 by American Journal of Neuroradiology