Graphical Abstract
Abstract
BACKGROUND AND PURPOSE: The determination of aneurysm wall enhancement (AWE) by human readers on visual inspection alone is subjective and prone to error. A 3D method for quantifying the signal intensity (SI) of the aneurysm enables objective determination of AWE. Interreader agreement and agreement between subjective and objective determination of AWE were assessed in this study.
MATERIALS AND METHODS: Patients with saccular intracranial aneurysms (IAs) were imaged with high-resolution MRI. In the subjective assessment, 2 internal adjudicators visually determined AWE if the degree of enhancement was equal to or higher than that of the pituitary stalk. An experienced internal neuroradiologist resolved disagreements. This internal adjudication was compared with an external adjudication to assess interrater agreement among centers. In the objective assessment, the distribution of SI across the aneurysm wall after normalizing the SI to the corpus callosum was determined with an in-house code. The normalized mean SI on postcontrast T1 MRI was defined as 3D-circumferential AWE (3D-CAWE). If the 3D-CAWE value was higher than 1, an IA was defined as objectively “enhancing.” Interrater agreement was analyzed with κ coefficients. Intertechnique agreement between the subjective and objective assessments was performed using κ statistics. Univariate regressions were used to identify which morphologic characteristics influenced subjective adjudication of enhancement.
RESULTS: A total of 113 IAs were analyzed. The agreement of the internal assessment was moderate (κ = 0.63), 49.5% of IAs (56) were classified as “enhancing;” and 50.5% (57) as “nonenhancing” after consensus. Interrater agreement between internal and external adjudication was weak (κ = 0.52) for the presence of AWE. There was no agreement between the subjective assessment of AWE and objective 3D-CAWE (κ = 0.16, P = .02). Subjective assessment was less likely to reliably adjudicate enhancement when assessing multiple aneurysms (OR, 0.4; 95% CI, 0.16–0.97; P = .04) and IAs larger than >7 mm (OR, 0.22; 95% CI, 0.09–0.55; P = .002) despite being objectively nonenhancing.
CONCLUSIONS: Subjective adjudication of AWE has poor interrater agreement, and no agreement with an objective 3D method of determining AWE. It is also less likely than objective quantification to identify enhancement in aneurysms of >7 mm or when multiple aneurysms are present. Objective 3D quantification, such as the technique used in this study, should, therefore, be considered when assessing AWE, especially in patients with multiple aneurysms and aneurysms of >7 mm in size.
ABBREVIATIONS:
- AWE
- aneurysm wall enhancement
- 3D-CAWE
- 3D circumferential aneurysm wall enhancement
- Gd
- gadolinium
- IQR
- interquartile range
- HR 3D T1 VWI
- high-resolution 3D T1-weighted black-blood vessel wall imaging
- HR-MRI
- high-resolution MRI
- IA
- intracranial aneurysm
- SI
- signal intensity
SUMMARY
PREVIOUS LITERATURE:
AWE is a potential biomarker of increased risk of aneurysm rupture. High-resolution MRI is currently used to evaluate AWE. However, the subjective assessment of AWE is prone to human error. While there is no universally accepted method for quantifying the degree of enhancement, several methods for objective quantification of enhancement have been proposed.
KEY FINDINGS:
Interrater agreement on subjective AWE is highly variable. A 3D method for quantifying AWE provides an objective assessment.
KNOWLEDGE ADVANCEMENT:
Subjective assessment of AWE is not only inconsistent between readers but also inconsistent when compared with a previously validated technique for objective 3D measurement of AWE.
Aneurysm wall enhancement (AWE) is a potential biomarker for identifying intracranial aneurysms (IAs) with increased risk of rupture.1 In a meta-analysis by Molenberg et al,2 which analyzed 1761 IAs, AWE was associated with rupture status, growth, or symptomatic presentation. According to a survey conducted by Mossa-Basha et al3 among members of the American Society of Neuroradiology, 52% of neuroradiologists use high-resolution MR imaging (HR-MRI) protocols in the analysis of the vessel wall. These protocols typically include pre- and postcontrast high-resolution T1-weighted black-blood sequences to assess enhancement, aiding in the differentiation of vasculopathies, determination of underlying atherosclerosis, and evaluation of aneurysms.
Expert consensus by Mandell et al4 recommended the use of HR-MRI in patients presenting with multiple IAs and SAH. However, accurate interpretation of HR-MRI relies on the reader’s experience,4 which may be prone to human error. Additionally, there is no consensus on the definition of AWE. Various definitions of AWE exist, including strong versus faint,5 focal or circumferential,6 and thin versus thick circumferential enhancement.7 Different methods for objective quantification of AWE using pre- and post-gadolinium (Gd) ratios have been proposed. A recent semiautomated method for 3D quantification of AWE allows the analysis of the distribution of the signal intensity (SI) of the aneurysm wall after normalization to the corpus callosum8 and the potential identification of symptomatic aneurysms with good accuracy (area under the curve = 0.914).9 We hypothesized that subjective assessment of AWE is unreliable, especially when interpreted by an inexperienced reader, and has poor agreement with the objective determination of enhancement. To test this hypothesis, we evaluated interreader agreement on subjective AWE adjudication, including agreement between experienced and inexperienced readers and agreement between subjective and objective determination of AWE. Additionally, we assessed factors that may impair the accuracy of subjective AWE assessment.
MATERIALS AND METHODS
This study was conducted according to The Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) initiative (Supplemental Data)10 and after approval from the institutional review board of University of Iowa. Patients with IAs underwent HR-MRI between May 2018 and November 2023. Inclusion criteria were patients with unruptured saccular IAs of >2 mm. Fusiform and thrombosed aneurysms were excluded. Demographic information was retrieved from the electronic medical records. Morphologic measurements of various IAs including size, size ratio, and aspect ratio were acquired from angiographic studies. Size was determined as the maximum diameter in the aneurysm.11 Size ratio was calculated as the maximal height divided by the average diameter of the parent vessels.12 Aspect ratio was calculated by dividing the aneurysm perpendicular height by the aneurysm neck width.13 Finally, aneurysm morphology was adjudicated as regular or irregular. An IA was considered irregular if it had daughter sacs or various lobes on different angiographic projections.14 Furthermore, the Population, Hypertension, Age, Size of aneurysm, Earlier subarachnoid hemorrhage, and Site of aneurysm (PHASES) and Earlier Subarachnoid Hemorrhage, Location of the Aneurysm, Age, Population, Size of the Aneurysm, and Shape of the Aneurysm (ELAPSS) scores were calculated.15
HR-MRI Protocol
Imaging was performed on a 3T scanner (Magnetom Skyra; Siemens). The protocol included TOF-MRA and a pre- and post-Gd sagittal 3D T1-weighted black-blood sequence (TE/TR, 900/15 ms; flip angle, variable; matrix, 320 × 320; FOV, 200 × 200; voxel size, 0.6 × 0.6 × 0.6 mm; slice thickness, 0.63 mm; acquisition time, 3 minutes 29 seconds). Post-Gd T1-weighted images were acquired 5 minutes after the administration of Gd (Supplemental Data).
Subjective Adjudication of Wall Enhancement
PACS was used to review and analyze the IA images. AWE was evaluated in multiple planes on high-resolution 3D T1-weighted black-blood vessel wall imaging (HR 3D T1 VWI) and subsequently adjudicated, as described by others.5⇓–7 The presence of enhancement was defined according to the criteria of Zhong et al:16 increased aneurysm wall SI equal or greater than the SI of the pituitary stalk on T1+Gd MRI and if it was not present before contrast administration. If AWE was observed, it was further classified as either “focal” or “circumferential” on the basis of the pattern of adjudication described by Edjlali et al.7 Focal enhancement was adjudicated if AWE involved only the neck, the dome, or a segment in the aneurysm wall, whereas circumferential enhancement was classified if the entire aneurysm wall enhanced. Two internal readers, each with at least 2 years of experience in interpreting HR-VWI, independently assessed the images for the presence and pattern of AWE. HR 3D T1 VWI is routinely included in the MRI protocol for patients with aneurysms at our institution, which is an academic center. Our radiologists follow the expert consensus guidelines of the American Society of Neuroradiology VWI study group when interpreting these studies.4 Disagreements between the 2 readers were adjudicated by an internal senior neuroradiologist with >10 years of experience in interpreting HR-VWI. This practice served as the “consensus internal adjudication.” In addition, an external neuroradiologist from a community center, who had no prior experience in interpreting HR-VWI, independently assessed the HR 3D T1 VWI for the presence of AWE (external adjudication). The external adjudicator received no specific training on the interpretation of HR 3D T1 VWI. This circumstance allowed assessment of variability in the interpretation of AWE on HR-VWI between experienced readers at an academic center and an inexperienced reader at a community center. All the adjudicators were blinded to any clinical information.
Objective 3D Quantification of AWE
As previously described, a 3D-AWE semiautomated method was used to quantify the SI of the aneurysmal wall in pre- and postcontrast HR 3D T1 VWI sequences.8,9 In the analysis of 93 aneurysms, this method achieved an area under the curve of 0.91 for detecting symptomatic aneurysms when combined with other variables such as age, aneurysm size, and female sex.9 Segmentations for each IA sac and the parent vessel were created using pre- and postcontrast HR 3D T1 VWI on 3D Slicer (5.6.1; http://www.slicer.org).17 To ensure accurate segmentation, we reviewed the 3D model of the aneurysm sac across multiple planes. Subsequently, a 3D hollow volume representing the aneurysm wall was generated for each aneurysm. A 3-mm ROI from the corpus callosum was used for normalization of the SI. No digital subtraction of the post-GD SI was performed. The 3D method uses the corpus callosum for SI normalization rather than the pituitary stalk because it is a more reliable structure for normalization8 due to minimal enhancement (<4%), its larger size (reducing partial volume effects that cause inaccurate measurement of SI), and more uniform signal (Supplemental Data). The pituitary stalk was used as a reference for subjective wall enhancement adjudication due to better visualization because it exhibits hyperintense signal relative to the cerebral white matter18 and normally enhances after Gd administration.19 Moreover, the pituitary stalk has been used extensively for normalization in most studies of AWE.18,20⇓–22 3D-AWE maps for each IA were generated using an in-house Matlab 2020a code (MathWorks; Supplemental Data). Multiple orthogonal spokes were then projected from the aneurysm sac into the wall to sample the SI of the entire aneurysm surface (Fig 1). The mean SI of the entire aneurysm wall in postcontrast HR 3D T1 VWI was obtained and defined as 3D circumferential AWE (3D-CAWE).8 In addition, histograms were generated from the 3D-AWE maps for quality control and better assessment of the distribution of enhancement within the aneurysm (Fig 1). An aneurysm was objectively considered “enhancing” if the 3D-CAWE (SI of the aneurysm body/corpus callosum) value was higher than 1.
3D-CAWE. The SI of the entire aneurysm wall is mapped from pre- and postcontrast HR 3D T1 VWI. 3D AWE colormaps represent the SI distribution in the aneurysm wall. In addition, histograms ensure that only the SI of the aneurysm wall is sampled and not surrounding structures like CSF or the cavernous sinus during the computation of 3D-CAWE. Finally, the mean SI in postcontrast HR 3D T1 VWI is calculated to determine 3D-CAWE (SI of the aneurysm body/corpus callosum ratio ≥1). A, A basilar tip aneurysm shows avid AWE after the administration of Gd. The ratio of AWE after normalization with corpus callosum ranges from 0 to 3. In this case, the basilar tip aneurysm is highly enhancing as demonstrated by the colormap (yellow indicates more enhancement). B, The histogram representation of the 3D-AWE colormap shows the distribution of SI across the aneurysm wall. The black curve is T1; and the red curve, T1+Gd. Note the shift of SI after Gd administration. The X axis indicates SI after normalization with the corpus callosum; and the Y axis, number of spokes that sample the aneurysm wall.
Statistical Analysis
Statistical analyses were performed using R Version 4.3.3 (http://www.r-project.org/). Normally distributed continuous variables are presented as mean (SD); and not normally distributed variables, as median and interquartile range (IQR). κ statistics were used to determine agreement23 among the following: 1) the 2 internal adjudicators, 2) the internal consensus adjudication and the external adjudicator, and 3) subjective assessment (internal consensus adjudication) and objective 3D CAWE assessment. The agreement between subjective and objective 3D-CAWE assessments was also determined. The impact of aneurysm location, multiplicity, clinical scales (PHASES and ELAPSS), and morphologic variables (size, size ratio, aspect ratio, and irregularity) on subjective adjudication was analyzed with univariate logistic regression. A multivariate model was constructed using the predictors from the previous univariate models. An all-subsets approach was used to find the optimal multivariate model as dictated by the Akaike Information Criterion. Predictor variables with correlation coefficients of >0.5 were not included in the same model.
RESULTS
A total of 92 patients with 113 IAs were included. Approximately 82% were women, and the mean age was 64 (SD, 12) years. The median IA size was 6.19 (IQR, 3.69); size ratio, 2.22 (IQR, 1.87); and aspect ratio, 1.17 (IQR 0.79, Table 1). Twenty-nine (32%) patients presented with symptoms at imaging; of these, 14% had oculomotor nerve palsy and 86% had a sentinel headache (Supplemental Data).
Demographic information of IAs (n = 113) according to objective quantification of wall enhancement
Subjective-versus-Objective Assessments of AWE
Internal interrater agreement was moderate (κ = 0.63) for the presence of AWE. However, there was no agreement regarding the pattern of enhancement (κ = 0.10). Following internal consensus adjudication, 56/113 (50%) IAs were defined as enhancing; and 57/113 (50%), as “nonenhancing.” Among the 56 IAs adjudicated as enhancing, 37 (66%) were deemed as having circumferential, and 19 (34%) focal enhancement. Interrater agreement on AWE between the internal consensus adjudication (academic center) and the external adjudication (community-based center) was weak (κ = 0.52) for the presence of AWE and minimal for the pattern (κ = 0.28) of enhancement. In the objective assessment, 19/113 (17%) IAs were classified as enhancing or 3D-CAWE+, while 94/113 (83%) were nonenhancing or 3D-CAWE–. The subjective and objective adjudications of AWE were significantly different (P = .02) and did not achieve agreement (κ = 0.16, Figs 2 and 3 and Table 2).
Subjective assessment of aneurysm AWE compared with the objective 3D-CAWE method. In clinical practice, AWE determination relies on visual adjudication, which is susceptible to bias. In the objective quantification of AWE, the mean SI on postcontrast HR 3D T1 VWI is normalized to the SI of the corpus callosum and is defined as 3D-CAWE. An IA is considered objectively enhancing if the CAWE value was >1. Aneurysms that are 3D-CAWE+ are in yellow; on the other hand, nonenhancing aneurysms or 3D-CAWE– are blue. A, Sagittal view of a left MCA IA subjectively adjudicated as enhancing and objectively, as 3D-CAWE+. B, Sagittal view of a basilar tip IA subjectively classified as enhancing; however, it was 3D-CAWE– on objective adjudication. C, Coronal view of the left supraclinoid ICA IA subjectively adjudicated as nonenhancing; nevertheless, it was objectively 3D-CAWE+. D, Sagittal view of a basilar tip IA subjectively described as nonenhancing and defined as 3D-CAWE. The histograms represent the mean SI on pre- and postcontrast HR 3D T1 VWI, which is used for determination of 3D-CAWE (SI of the aneurysm body/corpus callosum ratio of ≥1). CC indicates corpus callosum; Post, post administration of gadolinium.
Visual assessment of focal enhancement compared with 3D quantification of SI on the HR 3D T1 VWI sequence. In subjective AWE assessment, 2 patterns of enhancement are common: “circumferential” and “focal.” However, it remains to be determined if focal-versus-circumferential AWE is more likely to be correlated with aneurysm growth or rupture. The 3D-CAWE maps can highlight areas of focal enhancement. A, Sagittal view of an anterior communicating artery aneurysm shows “focal” enhancement (white arrow) on subjective assessment. In this case, despite having a very focal area of enhancement, the aneurysm is 3D-CAWE– (SI of the aneurysm body/corpus callosum ratio of ≤1) on histogram analysis. B, Similarly, a sagittal view of the basilar tip aneurysm has focal enhancement (white arrow) based on subjective determination; however, it is objectively “not-enhancing.”
Subjective adjudication of AWE compared with 3D quantification of SIa
Aneurysms of >7 mm (OR, 0.33; 95% CI, 0.15–0.72; P = .006) and those with higher size ratios (OR, 0.69; 95% CI, 0.49–0.95; P = .03) were less likely to be reliably adjudicated as enhancing by subjective assessment. In addition, adjudicators were less likely to precisely adjudicate AWE when assessing multiple IAs in the same patient (OR, 0.34; 95% CI, 0.12–0.85; P = .02) and aneurysms of >7 mm (OR, 0.32; 95% CI, 0.11–0.91; P = .04) compared with 3D-CAWE (Table 3 and Fig 4).
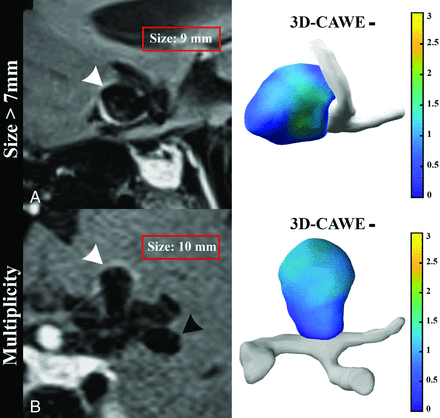
Aneurysm morphologic variables influencing experts’ assessment of AWE compared with 3D quantification. Experts are less likely to reliably adjudicate enhancement in aneurysm of >7 mm in size, and higher size ratio based on 3D-CAWE quantification. In addition, experts are less precise in characterizing enhancement when assessing patients with multiple IAs. A, Sagittal view of an anterior communicating artery aneurysm (white arrowhead) of 9 mm and 4.6 size ratio that is adjudicated as enhancing; however, it is objectively 3D-CAWE–. 3D colormap shows the absence of enhancement (blue). B, A patient with 3 intracranial aneurysms (right MCA aneurysm [not shown], left ICA terminus aneurysm [white arrowhead], and left MCA bifurcation [black arrowhead] aneurysm). A large left ICA terminus saccular aneurysm (10 × 6 mm) is visually adjudicated to have wall enhancement; however, it is categorized as objectively 3D-CAWE–. 3D colormap shows no enhancement (blue).
Univariate and multivariate regression analysis of aneurysm morphologic parameters associated with consensus adjudication between subjective and 3D objective quantification
DISCUSSION
This study aimed to assess the interrater agreement of subjective adjudication of AWE and its agreement with a previously validated objective 3D method for the determination of enhancement. The interrater agreement among adjudicators of the academic center for assessing AWE was moderate (κ = 0.63) and even lower compared with community-based adjudicators (κ = 0.52). There was minimal agreement between subjective and objective assessments of AWE. Adjudicators were less likely to precisely characterize AWE when assessing patients with multiple aneurysms and unruptured saccular IAs of >7 mm in size.
HR 3D T1 VWI can provide further insight into evaluating IAs, because AWE may serve as a surrogate biomarker for risk of rupture.24 Edjlali et al25 analyzed 108 IAs with HR-VWI and reported that AWE was the only factor associated with aneurysm instability (OR, 9.20; 95% CI, 2.92–29; P < .001). In clinical practice, AWE is determined by comparing pre- and postcontrast HR-VWI through direct observation, following expert consensus recommendations from the American Society of Neuroradiology.4 However, objective measures are required to gauge enhancement accurately. Consequently, different methods for SI estimation have been proposed.20 Interrater adjudication for AWE assessment is subject to variability. Our internal adjudication for the presence of AWE showed moderate agreement (κ = 0.63); while compared with the external assessment, it demonstrated weak agreement (κ = 0.52). Accurate interpretation of HR-VWI relies on reader experience,4 and in clinical studies, wall enhancement is commonly visually determined by 2 or 3 blinded adjudicators.26 This approach may be susceptible to observation bias. Moreover, structures near the aneurysm wall such as leptomeningeal vessels, brain parenchyma, vascular plexus, luminal thrombus, or microhemorrhages may lead to a false-positive results.27 Also, AWE adjudication may be prone to different interpretations due to the need for standardized definitions. Edjlali et al7 determined AWE by the thickness of the aneurysm wall that showed enhancement (>1 mm). Nagahata et al5 suggested classifying AWE as “strong” if enhancement was equal to or higher than that of the choroid or venous plexus and as “faint” if the wall SI increased compared with the precontrast T1 sequence. To potentially improve the subjective adjudication of AWE in our analysis, adjudicators compared the postcontrast HR 3D T1 VWI sequence with the pituitary stalk as a reference. Additionally, focal or circumferential enhancement patterns were defined on the basis of the criteria of Edjlali et al.7
However, there was minimal agreement among adjudicators on the enhancement patterns. Despite using standardized adjudication definitions, the subjective assessment of AWE remained inconsistent among trained academic and community-based adjudicators. Fu et al28 reported a high interrater agreement (κ = 0.87) for identifying AWE patterns and a strong interclass correlation coefficient (0.98) for measuring the wall enhancement index. The adjudicators’ extensive experience in neuroradiology, exceeding 20 years, likely contributed to the high concordance among raters. The weak agreement between the community-based radiologist and our internal consensus adjudication may be due to a lack of experience in interpreting HR 3D T1 VWI at the community center, where this technique is not routinely performed. Academic and community-based radiologists should be familiar with the normal appearance and variability of the vessel wall on HR-VWI, the patterns of enhancement after Gd administration, and the technical limitations and pitfalls in assessing AWE.
3D quantification of SI enables a more precise characterization of AWE distribution. Most groups have performed assessments of wall enhancement subjectively. Even the several “objective” methods of enhancement analysis are prone to bias because ROIs deemed enhancing are subjectively selected on 2D views. Sricharan et al developed a 3D quantification pipeline of SI capable of identifying areas of enhancement within the aneurysm wall (area under the curve = 0.74).29 Raghuram et al8 proposed a 3D method to map the SI distribution within the aneurysm wall after SI normalization to the corpus callosum, generating 3D colormaps of AWE to identify areas of increased SI. This method samples the aneurysm wall and generates thousands of data points (μ = 4490 spokes/aneurysm). Moreover, the quantification of the SI was previously optimized with 7T-MRI by tailoring the spoke length to the thickness of the aneurysm wall (range, 0.38–0.78 mm), decreasing potential artifacts introduced by nearby structures. These colormaps are correlated with histograms of enhancement from pre- and postcontrast HR 3D T1 VWI sequences (Fig 5).8 The subjective and 3D-CAWE methods of determining the presence of enhancement achieved minimal agreement (κ = 0.16). Molenberg et al2 suggested that the absence of AWE may indicate aneurysm stability, because growing, symptomatic, or ruptured IAs rarely do not exhibit AWE. Similarly, a metanalysis by Texakalidis et al30 reported that the absence of AWE was highly predictive of aneurysm stability (negative predictive value, 96.2%). However, using the presence of AWE to predict aneurysm growth or rupture is less reliable (positive predictive value, 55.8%). Subjective adjudication of enhancement may lead to more frequent follow-up imaging and treatment. Implementing 3D AWE methods eliminates subjectivity and mitigates variability resulting from subjective assessment of enhancement.
Enhancement versus artifact case. Basilar tip aneurysm with pseudoenhancement. A and B, Axial and sagittal views of a basilar tip aneurysm displaying false-positive enhancement on HR 3D T1 VWI. C, This aneurysm is not actually enhancing as demonstrated by the 3D colormap (blue indicates less enhancing). In this case, the anterior wall of the aneurysm sac is in close proximity to the pituitary stalk, therefore, looking as enhancing on axial (A) and saggital (B) views. The SI ratio of this aneurysm ranges between 0 and 1.6. D, The black curve represents T1, and the red curve represents T1+Gd. The 3D-CAWE value is lower than the objective 3D-CAWE (SI of the aneurysm body/corpus callosum ratio of ≥1), thus objectively labeled as nonenhancing.
Aneurysm morphology can influence the subjective adjudication of enhancement. Backes et al examined 89 unruptured IAs using HR-VWI and found that ≥7 mm in size was the primary determinant of Gd enhancement.31 However, HR-VWI may be affected by flow artifacts.32,33 Khan et al34 analyzed unruptured IAs on HR-VWI and discovered that larger aneurysms had lower blood flow rates, which may induce contrast stagnation.16 Slow flow can lead to inadequate SI suppression at the periphery of the aneurysm sac, simulating vessel wall thickening and pseudoenhancement.4,33 Identifying inadequate blood suppression is valuable for distinguishing pseudoenhancement caused by flow artifacts, both subjectively and objectively. Additionally, larger IAs are more likely to compress surrounding structures,35 and compromised veins can be mistaken for wall enhancement.36 We found that aneurysms of >7 mm in size (OR, 0.33; 95% CI, 0.15–0.72; P = .006) and with higher size ratio (OR, 0.69; 95% CI, 0.49–0.95; P = .03) were less likely to be reliably adjudicated as enhancing by raters.
Matouk et al36 found that HR-VWI could effectively detect the ruptured aneurysm in patients presenting with multiple IAs and SAH. In our study, we observed that adjudicators were less likely to precisely characterize enhancement when assessing patients with multiple aneurysms (OR, 0.4; 95% CI, 0.16–0.97; P = .04) and size >7 mm in size (OR, 0.22; 95% CI, 0.09–0.55; P = .002). Typically, the ruptured aneurysm can be determined on the basis of clinical signs, angiographic findings, aneurysm size and shape, focal hemorrhage on CT,37 and wall enhancement on HR-VWI.4 However, unruptured IAs may also show enhancement. 3D quantification of SI can aid in assessing AWE in patients with multiple IAs.
This study has several limitations. First, we lacked histologic validation to correlate objectively enhancing aneurysms with pathologic analysis. Second, the cross-sectional design of this study prevents us from assessing any causal relationships between AWE and subsequent treatment decisions, symptomatic status, or rupture outcomes. Third, we compared the subjective assessment of AWE with a comprehensive method that objectively measures the SI of the aneurysm wall. However, this latter method has not been validated as the criterion standard for determining enhancement. Fourth, inadequate blood suppression can increase the risk of pseudoenhancement, possibly confounding both subjective and objective assessments. However, histogram analysis from 3D color maps could be a useful tool for identifying false-positive enhancement determinations on CAWE. Finally, although we have a standardized protocol for acquiring HR 3D T1 VWI images 5 minutes after the administration of contrast, delays in acquisition may occasionally occur. However, such small delays would not affect the degree of enhancement of the pituitary stalk and compromise subjective assessment.
CONCLUSIONS
Subjective adjudication of AWE in saccular aneurysms is prone to bias and interreader variability. Objectively quantifying the SI of the aneurysm wall can improve AWE assessment. A 3D AWE method can be particularly useful for evaluating patients with multiple and larger aneurysms.
Acknowledgments
The authors thank The Brain Aneurysm Foundation for their support in funding this research.
Footnotes
This work was conducted on an MRI instrument founded by 1S10OD0250225-01 at the University of Iowa.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received June 13, 2024.
- Accepted after revision September 21, 2024.
- © 2025 by American Journal of Neuroradiology