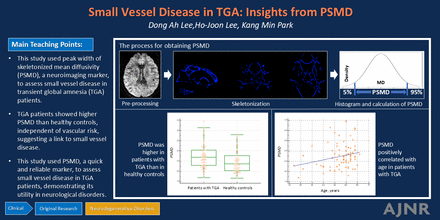
Graphical Abstract
Abstract
BACKGROUND AND PURPOSE: The peak width of skeletonized mean diffusivity (PSMD) is a novel marker of small vessel disease. In this study, we aimed to investigate the presence of small vessel disease in patients with transient global amnesia (TGA) by using the PSMD.
MATERIALS AND METHODS: We enrolled 75 patients newly diagnosed with TGA and included 65 age- and sex-matched healthy controls. DTI was performed by using a 3T MR imaging scanner. We measured the PSMD based on DTI by using the FSL program. This measure was compared between patients with TGA and healthy controls. Additionally, we conducted a correlation analysis to explore the relationship between PSMD and clinical factors.
RESULTS: A significant difference in the PSMD between patients with TGA and healthy controls was observed. Patients with TGA exhibited higher a PSMD compared with healthy controls (2.297 ± 0.232 versus 2.188 ± 0.216 × 10−4 mm2/s, P = .005). Additionally, patients with TGA but without any vascular risk factors, such as diabetes, hypertension, or dyslipidemia, also exhibited higher a PSMD compared with healthy controls (2.278 ± 0.253 versus 2.188 ± 0.216 × 10−4 mm2/s, P = .036). The PSMD positively correlated with age (r = 0.248, P = .032); however, it was not associated with duration of amnesia.
CONCLUSIONS: This finding underscores the feasibility of using PSMD as a marker for detecting small vessel diseases in patients with neurologic disorders. Furthermore, our study also implies the presence of small vessel disease may be present in patients with TGA.
ABBREVIATIONS:
- FA
- fractional anisotropy
- MD
- mean diffusivity
- PSMD
- peak width of skeletonized mean diffusivity
- TGA
- transient global amnesia
SUMMARY
PREVIOUS LITERATURE:
Transient global amnesia is a temporary neurologic condition characterized by retrograde and sometimes anterograde amnesia, typically resolving within 24 hours. Its pathophysiology is unclear, with hypotheses including arterial ischemia, venous congestion, migraines, epilepsy, and brain network abnormalities. TGA affects older adults, resembling transient ischemic attacks. While not generally linked to increased stroke risk, some studies suggest small vessel disease might contribute to TGA. A new marker, PSMD, has shown promise in assessing small vessel disease in other conditions, though it has not yet been applied to TGA research.
KEY FINDINGS:
Patients with TGA had significantly higher PSMD compared with healthy controls, even in those without vascular risk factors. PSMD also positively correlated with age but showed no association with the duration of amnesia, highlighting a potential link between TGA and small vessel disease.
KNOWLEDGE ADVANCEMENT:
This study used PSMD, a neuroimaging marker, to assess small vessel disease in patients with TGA. PSMD is quick to calculate, freely accessible, and has shown good interscanner reproducibility, highlighting its potential as a reliable marker for small vessel disease in neurologic disorders.
Transient global amnesia (TGA) is a paroxysmal neurologic disease with an incidence of approximately 5–10 cases per 100,000 people per year.1,2 TGA is primarily characterized by retrograde amnesia, with some cases also involving anterograde amnesia, and these symptoms typically resolve within 24 hours.1,2 TGA predominantly affects adults aged >50 years. While most patients experience only a single episode, some may have recurrent events.
Although the precise pathophysiology of TGA remains unclear, several hypotheses have been proposed, including arterial ischemia, venous congestion, migraine, epilepsy, and network abnormalities.3⇓⇓⇓⇓⇓-9 TGA typically occurs in older people, presents temporarily, and then resolves, which is similar to a TIA. In addition, it often shows high signal intensity on DWI MRI in the hippocampus, particularly in the CA1 region. This observation suggests that arterial ischemia may be a contributing factor to the pathophysiology of TGA.3 Alternatively, the Valsalva maneuver by strenuous physical activity increases intrathoracic pressure and can lead to transient reductions in blood flow to the brain by impacting the venous return to the heart. It is known to trigger TGA events, and the higher prevalence of internal jugular valvular insufficiency observed in patients with TGA suggests that venous congestion might be a contributing factor for TGA attack.4 Moreover, the transient nature of TGA symptoms may indicate a connection to migraines or epilepsy.5,6 Recently studies on brain connectivity in patients with TGA have revealed abnormalities in brain networks, including the default mode network and limbic network, which may contribute to TGA pathophysiology.7⇓-9
While cerebral ischemia is considered a potential cause of TGA, the role of small vessel disease in the pathophysiology of TGA remains controversial. Multiple case-control studies have found no significant difference in the prevalence of vascular risk factors, such as hypertension, diabetes mellitus, and dyslipidemia, between patients with TGA and age- and sex-matched controls.10,11 However, 1 study based on a National Inpatient Sample including 58 million hospital cases found that patients with TGA were almost twice as likely to have hypertension and 3 times as likely to have dyslipidemia compared with the overall inpatient population.12 A large comparative study involving patients with TGA, patients with TIA, and matched controls reported that patients with TGA are more likely to have dyslipidemia, a history of ischemic stroke, and ischemic heart disease compared with age- and sex-matched controls. Nonetheless, compared with patients with TIA, those with TGA are less likely to have hypertension, diabetes mellitus, a history of ischemic stroke, and atrial fibrillation.13 In general, TGA is not associated with an increased risk of ischemic stroke.14,15 However, a recent nationwide study has reported that TGA could be an important risk factor for ischemic stroke.16 Additionally, a recent MRI study involving 69 patients with TGA has shown that the burden of small vessel disease is higher in patients with TGA than in healthy individuals.17 Therefore, further research on the relationship between TGA and small vessel disease is should be conducted.
Recently, the peak width of skeletonized mean diffusivity (PSMD) has been developed as an objective marker for investigating small vessel disease.18 This is a neuroimaging marker that is automatically calculated based on DTI and expressed as a number. DTI is a sensitive technique that allows quantifying microstructural tissue changes, and DTI metrics, such as fractional anisotropy (FA) and mean diffusivity (MD), are superior to conventional imaging markers in assessing disease burden in small vessel disease.19,20 However, DTI requires extensive data postprocessing, particularly the removal of prominent CSF signals from MD images. The PSMD can be easily used in clinical routine practice and can apply to large samples. The skeletal map of MD eliminates CSF contamination, and the histogram-based approach enhances the ability to capture subtle diffuse disease features.18 Further, the PSMD has shown a good interscanner reproducibility.21 This measure has been used to examine small vessel disease in various neurologic diseases, such as Alzheimer disease, Parkinson disease, and multiple sclerosis, demonstrating its potential as a more reliable marker than conventional markers.22⇓⇓⇓-26 The PSMD has outperformed traditional DTI parameters in predicting cognitive variability.18,21,25,27,28 Notably, unlike mean MD and FA, PSMD reflects a measure of dispersion rather than central tendency, capturing the heterogeneity of MD values across the white matter skeleton. PSMD may be more sensitive to regional MD variability by encompassing multiple sources of diffusion heterogeneity, which could account for its superior performance compared with conventional DTI markers, such as average MD.18,21,25,27,28 However, it has not yet been used to investigate small vessel disease in patients with TGA.
In this study, we aimed to investigate the presence of small vessel disease in patients with TGA by using PSMD. We hypothesized that TGA could be associated with small vessel disease, which may correlate with the clinical symptoms of TGA. This article follows the Strengthening the Reporting of Observational studies in Epidemiology reporting guidelines.
MATERIALS AND METHODS
Participants
This cross-sectional study was retrospectively conducted at a single tertiary hospital and was approved by the institutional regional board of the hospital. We enrolled patients with TGA by using the following criteria: 1) newly diagnosed TGA at our hospital with a clinical history consistent with the condition,1,2 including anterograde amnesia, no clouding of consciousness or loss of self-identity, cognitive impairment limited to amnesia, and no focal neurologic or epileptic signs, 2) DTI conducted at the time of TGA diagnosis, 3) no structural lesions on brain MRI, except hippocampal dot lesions on DWI, and 4) no epileptiform discharges on electroencephalography. DTI data underwent visual inspection to exclude artifacts, and patients with artifacts were excluded from this study. Among the patients with TGA, we also investigated those who had been previously diagnosed with diseases of vascular risk factors such as diabetes, hypertension, or dyslipidemia.
We also included age- and sex-matched healthy controls who had already been enrolled as the normal group in our previous study. They had normal brain MRI and no other medical, neurologic, or psychiatric diseases. They did not have any vascular risk factors, such as diabetes, hypertension, or dyslipidemia.
We obtained the clinical characteristics of the patients with TGA, such as age, sex, duration of amnesia, precipitating factors, medical history, and hippocampal dot lesions on DWI.
DTI Scan
All MRIs were acquired at the time of TGA diagnosis. All MRI scans were performed by using a 3T MRI scanner (AchievaTx; Phillips Healthcare) equipped with a 32-channel head coil for both patients with TGA and healthy controls. High-quality 3D T1-weighted imaging, 3D FLAIR imaging, coronal T2-weighted imaging, axial DWI, and DTI were conducted, which were routine MRI protocols for patients with TGA at our hospital. The FLAIR and T2-weighted imaging were used to investigate the structural lesions in patients with TGA. The scans utilized spin-echo single-shot echo-planar pulse sequences with 32 different diffusion directions (TR/TE, 8620/85 ms; flip angle, 90°; slice thickness, 2.25 mm; acquisition matrix, 120 × 120; field of view, 240 × 240 mm2; and b-value, 1000 seconds/mm2).
Obtaining the PSMD
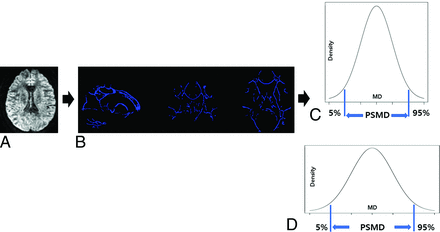
We obtained the PSMD from DTI by using the FSL program (http://www.fmrib.ox.ac.uk/fsl) installed on a Linux system, involving a total of 4 steps (Fig 1).18 The preprocessing the DTI data involves several methodical steps. Initially, the diffusion tensor images were processed by using the eddy_correct tool with the FSL default settings, followed by brain extraction and tensor fitting. Skeletonization is performed by using tract-based spatial statistics, where an FA map was registered to a common space and projected onto a skeleton. The same transformation matrices were then applied to the MD data to generate a skeletonized MD map. Subsequently, a custom mask was applied, which utilized a template thresholded at an FA value of 0.3 along with an additional custom-made mask to prevent contamination of the skeleton by CSF. Finally, histogram analysis was conducted, wherein the width of the histogram derived from the MD values of all voxels within the skeleton was measured. The PSMD was calculated as the difference between the 95th and 5th percentiles.
The process for obtaining PSMD. Preprocessing for DTI, including motion and eddy current correction, brain extraction, and tensor fitting (A). Skeletonization, including normalization, projection to the skeleton template, and application of a custom mask (B). Histogram analysis and calculation of PSMD from the difference between the 95th and 5th percentiles in healthy controls (C) and patients with transient global amnesia (D).
Statistical Analysis
The primary outcome measure of this study was the PSMD. No statistical power calculation was performed before this study. The sample size was based on the available data. An independent sample t test was used to compare age and PSMD values between patients with TGA and healthy controls. The χ2 test was used to compare sex differences between the groups. Pearson correlation test was used for correlation analysis. Statistical significance was considered when the P value ≤ .05. All statistical analyses were performed by using MedCalc Statistical Software Version 22.009 (MedCalc Software; https://www.medcalc.org; 2023).
RESULTS
Demographic and Clinical Characteristics of Participants
We enrolled 75 patients newly diagnosed with TGA and 65 healthy controls. Tables 1 and 2 list the demographic and clinical characteristics of patients with TGA and healthy controls. Age and sex were comparable between the patients with TGA and healthy controls. Of the 75 patients with TGA, 65 experienced a single TGA event and 10 experienced recurrent TGA events. Additionally, 19 patients with TGA had vascular risk factors, such as diabetes mellitus, hypertension, or dyslipidemia.
Demographic and clinical characteristics of patients with TGA and healthy controls
Demographic and clinical characteristics of patients with TGA and healthy controls
Difference in the PSMD between the Groups
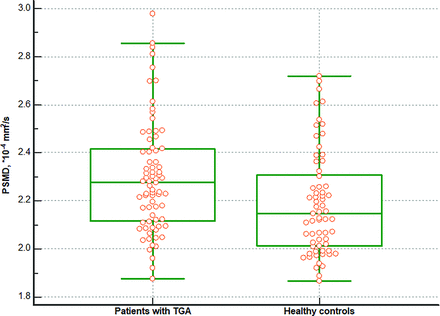
There was a significant difference in the PSMD between patients with TGA and healthy controls. The patients with TGA exhibited higher PSMD compared with healthy controls (2.297 ± 0.232 versus 2.188 ± 0.216 × 10−4 mm2/s, P = .005) (Fig 2). Additionally, patients with TGA but without any vascular risk factors also exhibited higher PSMD compared with healthy controls (2.278 ± 0.253 versus 2.188 ± 0.216 × 10−4 mm2/s, P = .036). However, there was no difference in the PSMD based on the recurrence of the TGA attack. The PSMD in patients with a single TGA event did not differ from that in patients with recurrent TGA events (2.296 ± 0.223 versus 2.299 ± 0.297 × 10−4 mm2/s, P = .976).
Difference in the PSMD between patients with TGA and healthy controls. The PSMD was higher in patients with TGA than in healthy controls (2.297 versus 2.188 × 10−4 mm2/s, P = .005).
Correlation between the PSMD and Clinical Characteristics
In patients with TGA, the PSMD was positively correlated with age (r = 0.248, P = .032) (Fig 3). However, it was not associated with the duration of amnesia (r = −0.114, P = .380). The PSMD was also correlated with age in healthy controls (r = 0.324, P = .008).
Correlation analysis between age and PSMD in patients with TGA. PSMD positively correlated with age (r = 0.248, P = .032) in patients with TGA.
DISCUSSION
Our study revealed that patients with TGA with or without vascular risk factors exhibited a higher PSMD compared with healthy controls, suggesting a significant association between small vessel disease and TGA. Additionally, we found that the PSMD increased with age, implying that small vessel disease increased with age.
Our present results, suggesting small vessel disease in patients with TGA, are supported by the results of previous studies. Wang et al17 examined imaging markers of small vessel disease in 69 patients with TGA and 69 healthy controls. They revealed that the burden of small vessel disease, including lacunes, white matter hyperintensities, and enlarged perivascular space, was higher in patients with TGA than in healthy controls.17 Interestingly, they also showed that the burden of this small vessel disease was higher in the group with recurrent TGA events.17 Furthermore, a study of 35 patients with TGA demonstrated that those with hippocampal dot lesions on DWI exhibited significantly higher rates of carotid atherosclerosis.29 In addition, a study of 372 hospitalized patients with TGA showed that female patients with TGA had significantly higher systolic blood pressure and a higher degree of cerebral microangiopathy, as evaluated by a neurologist by using Fazekas score upon admission.30 Other studies have found that patients with TGA had high prevalence of vascular risk factors, such as hypertension and dyslipidemia, compared with the corresponding healthy controls.12,31 These findings suggest the evidence of small vessel disease in patients with TGA.
The pathogenic mechanism underlying the association between TGA and small vessel disease remains unclear. The high prevalence of small vessel disease in patients with TGA might be attributed to several factors. One possible reason for this is that small vessel disease is often associated with conditions such as hypertension and diabetes, which can predispose individuals to TGA. However, in this study, we found that the PSMD was higher in patients with TGA, even in the absence of vascular risk factors such as hypertension or dyslipidemia, than in the normal group. This indicates that TGA is associated with small vessel disease independent of the vascular risk factors. Another assumption is that microvascular changes and reduced cerebral perfusion related to small vessel disease may make the limbic structures of the brain more susceptible to TGA episodes. Small vessel disease can contribute to transient disruptions in the cerebral blood flow, leading to TGA. A previous voxel-based morphometry study also demonstrated that the volume of limbic structures was reduced in patients with TGA compared with that in controls.32 Further research is required to understand the mechanisms linking small vessel disease and TGA fully.
A previous study with a large sample size showed a significant association between age and PSMD, consistent with our findings.28 Our study also confirms that small vessel disease increases with age in patients with TGA. The underlying mechanisms of small vessel disease with aging may be multifactorial. Aging impairs the function of the endothelium and inner lining of brain blood vessels.33 This endothelial dysfunction can reduce the ability of vessels to dilate properly and respond to changes in blood flow, increasing the risk of ischemia and microvascular damage. Additionally, oxidative stress and chronic inflammation increase with age, exacerbating vascular injury.33 Aging is also linked to the breakdown of the blood–brain barrier, which normally protects the brain from harmful substances in the bloodstream.34 This breakdown can cause blood components to leak into the brain tissue, contributing to vascular damage. Furthermore, genetic predispositions and epigenetic changes associated with aging can influence the development and progression of small vessel diseases.35
In this study, we used the PSMD, a neuroimaging marker, to examine small vessel disease in patients with TGA. The PSMD is well correlated with conventional MRI markers of small vessel disease, such as white matter hyperintensities, lacunes, and enlarged perivascular space.21,27,36 It provides an objective evaluation of small vessel disease severity due to its automatic measurement capabilities.18 In addition, the PSMD can be calculated quickly, and the method for calculating it is widely available for free. Previous studies have already demonstrated that the PSMD has good interscanner reproducibility.18,21
To our knowledge, this study is the first to investigate small vessel disease in patients with TGA by using PSMD and successfully provides evidence of such disease in this population. However, this study has some limitations. First, it is a retrospective study conducted at a single tertiary hospital. Thus, selection bias may inevitably occur during the enrollment of patients with TGA. Second, the cross-sectional design and the fact that the patients underwent MRI after visiting the hospital post-TGA event make it challenging to establish a cause-and-effect relationship between small vessel disease and TGA. Third, we could not investigate or control for factors such as smoking and alcohol consumption. Despite these limitations, the study provides a clear evidence base for the presence of small vessel disease in patients with TGA.
CONCLUSIONS
This finding underscores the feasibility of using PSMD as a marker for detecting small vessel diseases in patients with neurologic disorders. Furthermore, our study also implies small vessel disease may be present in patients with TGA.
Footnotes
Dong Ah Lee and Ho-Joon Lee contributed equally to this study.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT)(No. RS-2023-00209722).
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received August 12, 2024.
- Accepted after revision October 6, 2024.
- © 2025 by American Journal of Neuroradiology