SUMMARY:
Hyrtl fissure is a rare cause of congenital CSF otorrhea in infants, with only a few cases previously described in the English literature. Given the predisposition to repeated ear and intracranial infections, identification of this bony anomaly is critical in the diagnosis and management of recurrent meningitis or otitis media in a child, especially in the presence of CSF otorrhea. We describe 3 cases of this entity with a specific focus on the embryologic basis of the etiology and conventional and advanced imaging features of this unique entity.
Hyrtl fissure, also known as a tympanomeningeal fissure, is a normal anatomic site in the embryologically developing temporal bone.1 This fissure is normally walled off through natural ossification by gestational week 24.2,3 However, in some cases, incomplete or absence of ossification can lead to a variation where the fissure remains patent. In such patients, a fistula between the subarachnoid space and the tympanomastoid cavity resulting from the patent fissure can cause CSF otorrhea and create a pathway for infection, either in the form of otitis media or meningitis.1,4 Prompt identification and treatment of this entity is essential to preventing a severe infection that could impede hearing and neurocognitive development. In the 3 patients discussed below, we describe the symptomatic presentation, imaging features, treatment performed, and subsequent outcomes.
CASE SERIES
Case 1
A 1-year-old female child presented with irritability and an ear infection. Her prior history was relevant for multiple episodes of ear drainage, ear infections, and recurrent meningitis. A tympanostomy tube was placed, and examination of the fluid was positive for β-2 transferrin confirming CSF otorrhea.
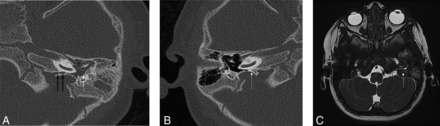
CT of the temporal bones was then performed that demonstrated a thin left-sided bony cleft, consistent with Hyrtl fissure, communicating with the opacified left middle ear cavity (Fig 1A, -B). An MRI of the temporal bones was also performed that clearly demonstrated a CSF-containing cleft between the middle ear cavity and the subarachnoid space of the posterior fossa (Fig 1C).
HRCT of the temporal bones (A and B) demonstrating a thin bony cleft, consistent with a Hrytl fissure (black arrows), communicating with the opacified left middle ear cavity (white arrow). This is in comparison to the contralateral side, where no bony cleft and normal ossification is seen (white arrow). Axial T2-weighted high resolution CISS image (C) clearly demonstrating the cleft with CSF signal intensity, between the middle ear cavity and the subarachnoid space of the posterior fossa. (white arrow).
The child underwent surgery which involved raising the tympanomeatal flap to enter the middle ear cavity followed by middle ear exploration and plugging of the defect. She continues to be followed by neurosurgery and otolaryngology and has not presented with meningitis since the surgery.
Case 2
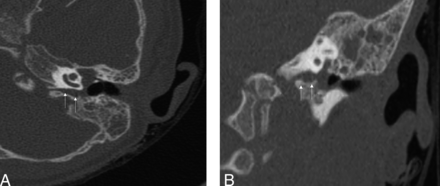
A 23-month-old female patient with a history of chronic left-sided otitis media presented to her ENT surgeon for a scheduled placement of a tympanostomy tube. Upon myringotomy, clear fluid was seen to gush out from the middle ear cavity. At least 8 mL of the fluid was collected, and laboratory evaluation confirmed CSF otorrhea. CT scan of the temporal bones revealed a large fluid filled cleft, between the bony labyrinth and jugular foramen, resulting in communication between the subarachnoid space of the posterior fossa and the mesotympanum (Fig 2). The left middle ear cavity and mastoid air cells were opacified. No abnormalities of the tegmen, facial nerve, or the inner ear structures were noted. A day later, the patient presented to the emergency department with fever and chills, lethargy, nausea. and vomiting. A lumbar puncture confirmed the diagnosis of meningitis. The child was operated via the middle ear fossa approach, with drilling of the fissure to remove the mucosa and packing of the defect.
Axial (A) and coronal (B) images from a temporal bone CT demonstrating a large fluid attenuating cleft between the bony labyrinth and jugular foramen, resulting in communication between the subarachnoid space of the posterior fossa and the mesotympanum. The left middle ear cavity and mastoid air cells were opacified.
Case 3
A 4-month-old male infant was hospitalized with otorrhea and bacterial meningitis due to H influenza. The patient had had multiple prior episodes of meningitis in the first few months of life. A CT scan of the temporal bones was performed that demonstrated the Hyrtl fissure (Fig 3A). The left middle ear cavity and petrous bone were opacified, containing CSF (Fig 3B, -C). MR images demonstrated CSF within the left petrous apex and the Hyrtl fissure containing CSF. The patient also had an MR myelogram that nicely demonstrated the fissure filled with intrathecal contrast (Fig 3D). He underwent surgery for plugging of the defect and is currently doing well.
Bone window from axial CT of temporal bone (A) demonstrating a Hrytl fissure in the left temporal bone (black arrow). Axial T2-weighted (B) and FLAIR (C) MR images demonstrating fluid signal intensity within the middle ear and the left petrous apex as seen on the T2-weighted image which suppresses on the T2 FLAIR sequence suggesting CSF. Coronal MR myelogram T1-weighted image (D) confirming the fissure filled with intrathecal contrast (white arrow).
DISCUSSION
Congenital CSF otorrhea results from a fistulous connection between the subarachnoid space of the brain and the tympanomastoid cavity. This may occur through an abnormal labyrinth such as a Mondini dysplasia or through a pathway distant from a normal labyrinth.3,5 Causes of fistulas include widening of normal inner ear structures such as the petromastoid canal, the facial canal, or the cochlear aqueduct, or defects in the tegmen tympani and the tympanomeningeal (Hyrtl) fissure.3,5
Hyrtl fissure is a congenital, infralabyrinthic cleft and a rare cause of a CSF fistula. It lies within the primitive cochlear aqueduct, a compartment that communicates with the posterior cranial fossa located medial to the round window niche. In addition to Hyrtl fissure, the perilymphatic duct and inferior cochlear vein also reside within the primitive cochlear aqueduct.6 In normal gestational development, the bony structures surrounding the Hyrtl fissure ossify by week 18, with the lateral margin of the round window being nearly fully formed.4 At the same time, the portion medially onto which the round window membrane attaches, develops as cartilaginous tissue with subsequent ossification by week 24.4,5,7⇓–9
The Hyrtl fissure turns into the lateral wall of the cochlear aqueduct and remains closed from this point onward in typical development. In some instances, for reasons unknown, complete ossification of the medial rim may not take place near the 24th week of gestation, resulting in a patent fissure that persists postnatally as a permanent peri labyrinthine fistula.1,6 The fissure results in a direct communication between the subarachnoid space of the brain and the middle ear cavity. Initially children usually present with signs of recurrent middle ear infection, which may lead to meningitis, due to direct communication with the subarachnoid space of the brain.8 Sometimes increased middle ear pressure might lead to tympanic membrane perforation, resulting in CSF otorrhea as a presenting symptom. Less common presentations include detection during cochlear implant planning or placement or in association with other developmental anomalies.8
High-resolution CT of the temporal bones is the mainstay imaging technique in patients with CSF otorrhea. Because the most common route for congenital CSF otorrhea is translabyrinthine, the morphology of the inner ear structures, particularly the bony labyrinth, should be evaluated thoroughly. Although tegmen tympani/mastoideum defects are a more common cause of CSF otorrhea in adults, sometimes they might occur in children and should be looked for as well. In cases when the Hyrtl fissure is a cause of the CSF otorrhea, a cleft between the tympanic cavity and the posterior fossa can be seen. The fistulous communication might be air attenuation or fluid attenuation, if opacified with CSF. The middle ear cavity may be seen opacified with an effusion as well. On MR, a CSF-containing cleft between the tympanic cavity and the subarachnoid space of the posterior fossa can be seen. Middle ear or a mastoid effusion when present can be easily identified on the T2-weighted images. Imaging findings of meningitis in the brain can be seen, in cases of intracranial spread of infection. Sometimes, fistulous communication might not be easily identified or missed. In such cases, a CT or MR myelogram is useful in the work-up. In cases of a patent fissure, contrast opacification of the middle ear cavity, subarachnoid place of the posterior fossa and the cleft can be seen, thereby helping make a definitive diagnosis.
Once identified, treatment of the defect usually requires surgical management. Surgical approaches for repair include endaural, transcanal, retrosigmoid, postauricular, and posterior fossa endoscopic.8 Transcanal middle ear exploration is the most direct way of visualizing the fissure and obliteration of the fissure can be achieved with osteoconductive material or other composite materials such as fibrin glue or ceramic material.2,8
CONCLUSIONS:
Congenital CSF otorrhea is a relatively rare entity and has been described to occur due to various etiologies in the setting of an abnormal as well as normal labyrinth. CT and/or MR imaging is essential in further work-up of CSF otorrhea and may be helpful in identifying a potentially treatable cause. Hyrtl fissure is a rare and surgically treatable cause of CSF otorrhea, diagnosis of which can be made on CT of the temporal bones or MR of the skull base/IAC. Rarely, the fissure might not be seen on cross-sectional imaging. In such cases, particularly if there is high suspicion, further imaging with a CT or MR myelogram might be warranted. Surgical closure of the fissure is the mainstay of treatment for these patients, with a high rate of success.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received July 16, 2024.
- Accepted after revision August 30, 2024.
- © 2025 by American Journal of Neuroradiology