Jump to comment:
- Page navigation anchor for RE: Response to e-Letter: Low ADC in the Cerebellum of Infants: A Normal Developmental Phenomenon RE: Norrie Disease: Cochlear Enhancement and Cerebellar Signal Abnormalities.RE: Response to e-Letter: Low ADC in the Cerebellum of Infants: A Normal Developmental Phenomenon RE: Norrie Disease: Cochlear Enhancement and Cerebellar Signal Abnormalities.
We thank Drs. Agripnidis and Hak for their thoughtful critique of our recent publication and for highlighting the importance of age-specific interpretation of diffusion-weighted imaging (DWI) of the cerebellum in infants.
Their observations raise important points regarding normal cerebellar maturation, which we agree must inform interpretation of diagnostic brain MRIs in early infancy. However, we believe that the cerebellar abnormalities presented in our Norrie Disease (ND) cases reflect a pathologic process beyond expected developmental change.
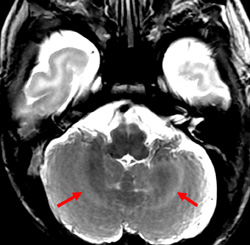
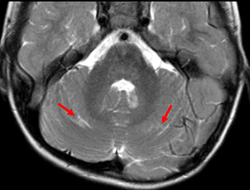
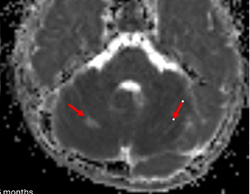
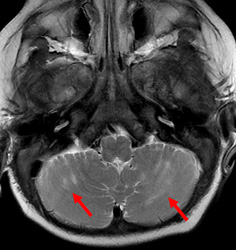
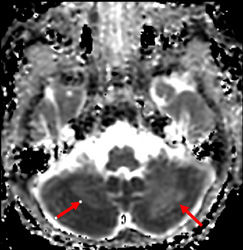
Importantly, we would like to provide additional clinical and imaging information that was not included in our original manuscript for reasons of brevity. In both patients, cerebellar signal abnormality on T2-weighted images was present at presentation (Figs 1, 4, and 5), along with reduced ADC values, and cerebellar enhancement. In Case 1 (the 5-week-old), a follow-up MRI at 3 years and 6 months demonstrated chronic evolved cerebellar injury (Fig 2 and 3).
Additionally, the cerebellar ADC abnormalities in both infants were first detected through subjective visual inspection and subsequently confirmed by quantitative analysis. This diagnostic approach reflects standard clinical practice and emphasizes the contribution of visually recognizing apparent, regionally specific signal abnormalities, particularly in genetically confirmed neurovascular conditions.
Quantitative ADC measurements from the initi...
Show MoreCompeting Interests: None declared. - Page navigation anchor for Low ADC in the Cerebellum of Infants: A Normal Developmental PhenomenonLow ADC in the Cerebellum of Infants: A Normal Developmental Phenomenon
I would like to thank the authors for these new data on Norrie disease. However, we believe an important point deserves further discussion: the quantitative evidence of low ADC in the neonatal cerebellum in infants under 6 months, ADC values in the cerebellum can physiologically fall around 0.6–0.7 × 10⁻³ mm²/s due to normal maturation processes. Data from Özkan et al. (2019)(1) show that the mean ADC in the cerebellar cortex reaches as low as 0.75, with standard deviations allowing lower bounds down to 0.63. Similarly, the middle cerebellar peduncle shows values dropping to 0.78 with normal variability reaching 0.66. These findings confirm that ADC values near 0.6–0.7 are part of normal cerebellar development, not pathological restriction. (Table 1)
Show More
These lower values typically occur during the first few weeks to months after birth and reflect rapid maturation processes rather than diffusion restriction due to injury.
There are several mechanisms behind physiological ADC reduction.
First, early myelination: the cerebellar white matter and peduncles myelinate early, often from birth to 3 months of age on T1-weighted imaging(2). Myelination reduces extracellular water, restricts water diffusion, and lowers ADC.
Second, high cellular density: The cerebellum has a persisting external granular layer in the early postnatal period. This temporary, densely packed layer leads to reduced interstitial space and further restricts diffusion physiologically....Competing Interests: None declared.