SUMMARY:
Spontaneous intracranial hypotension is an increasingly recognized syndrome caused by a spinal CSF leak, with most reported cases occurring in adults. The use of specialized or advanced myelography to localize spinal CSF leaks has evolved substantially in recent years, particularly since the initial description of CSF-venous fistulas in 2014. To our knowledge, no prior series have evaluated the use of specialized myelographic techniques to localize CSF leaks in children with spontaneous intracranial hypotension, likely because the disease is rare in this patient population. This issue may be compounded by a hesitation to perform invasive procedures in children. In this clinical report, we conducted a multi-institutional review of pediatric patients with spontaneous spinal CSF leaks localized using advanced myelographic techniques, such as prone and decubitus digital subtraction and CT myelography, as well as dynamic CT myelography. We report the clinical features of these patients, as well as imaging findings, types of leaks discovered, and method of treatment. We found that the primary types of spontaneous spinal CSF leaks that occur in adults, including dural tears and CSF fistulas, can be seen in children, too. Furthermore, we show that specialized myelographic techniques can successfully localize these leaks and facilitate effective targeted treatment.
ABBREVIATIONS:
- CTM
- CT myelography
- CVF
- CSF-venous fistula
- DSM
- digital subtraction myelography
- ICHD
- International Classification of Headache Disorders
- SIH
- spontaneous intracranial hypotension
Spontaneous intracranial hypotension (SIH) is a clinical syndrome that is almost always caused by a spinal CSF leak. In recent years, knowledge regarding the types of CSF leaks that cause SIH has evolved substantially. One commonly used classification system divides spinal CSF leaks into ventral dural tears (type 1a), posterior/lateral dural tears (type 1b), leaking meningeal diverticula (type 2), and CSF-venous fistulas (CVFs, type 3).1 Although the diagnosis of SIH can be made on the basis of symptoms and brain MRI findings, advanced myelographic techniques such as digital subtraction myelography (DSM), dynamic CT myelography (CTM), or decubitus CTM without a dynamic component are typically needed to determine the exact type and location of the underlying spinal CSF leak.2,3 These types of specialized or advanced myelography differ from conventional CTM insofar as images are typically obtained more quickly after contrast injection and patient positioning is maintained after injection to prevent dilution of contrast throughout the subarachnoid space. Because all leak types usually have similar clinical presentations related to the common pathway of unregulated CSF volume loss and spinal MRI and conventional CTM can only rarely localize spinal CSF leaks, advanced myelography with a proper technique and patient positioning is typically necessary for accurate diagnosis and localization.
Most cases of SIH occur in adults. However, SIH can occur in children as well. The largest series of pediatric patients with SIH was described by Schievink et al4 in 2013. This was a comprehensive study illustrating clinical presentations, as well as brain MRI, spinal MRI, and conventional CTM findings in 24 children. Since that time, the various specialized myelographic techniques to localize CSF leaks more precisely have been developed. To our knowledge, examples of pediatric spinal CSF leaks localized using modern myelographic techniques have been limited to individual case reports. This is likely because SIH in children is quite rare, and leaks that have been localized on myelography are rarer still. We conducted a retrospective, multi-institutional review of cases of SIH in children whose leaks were precisely diagnosed and localized on advanced myelography, such as prone/decubitus DSM, decubitus CTM, and prone/decubitus dynamic CTM. Our goal was to describe the various etiologies of SIH present in children and elucidate the potential benefits of advanced myelography in establishing a diagnosis and permitting targeted treatment.
CASE SERIES
Materials and Methods
The study was deemed exempt by Mayo Clinic institutional review board. The radiologic databases from 5 different institutions specializing in the diagnosis and treatment of SIH were queried. The date range for inclusion spanned 8 years (January 2016 through May 2024), with the starting date corresponding to the establishment of modern myelographic techniques at participating institutions. Inclusion criteria were the following: 1) diagnosis of SIH using the International Classification of Headache Disorders (ICHD-3) criteria, 2) age younger than 18 years at the time of symptom onset, and 3) CSF leak precisely localized by using advanced myelography, including decubitus CTM, prone/decubitus DSM, or prone/decubitus dynamic CTM.5 We excluded any patients with nonspontaneous (ie, iatrogenic or posttraumatic) CSF leaks.
Clinical information for each patient was obtained from the medical record, including presenting symptoms, pertinent history, and details of any known genetic or other syndromes. Brain MRI and spine MRI findings were reviewed by 1 radiologist from each institution. Note that the SIH Bern score was not recorded, because this system was initially validated in an adult-only cohort.6 Instead, we assessed the presence of subjective brain sagging, pachymeningeal enhancement/thickening, and/or venous sinus distention.
Myelographic images were reviewed by the performing radiologist at each institution to confirm the site, level, and type of leak. An additional radiologist reviewed each study independently to confirm the findings. All reviewing physicians were board-certified, practicing neuroradiologists. Each radiologist reviewing the studies had subspecialty expertise in performing and interpreting advanced myelography for patients with spinal CSF leaks. Clinical information from each patient was obtained, including a history of any connective tissue disorder or specific heritable syndrome, presenting symptoms, the presence of any major inciting factor before symptom onset, details of leak treatment, and posttreatment follow-up information.
Results
Among the 5 participating institutions, we identified 12 pediatric patients with SIH who had myelographically localized spinal CSF leaks. No patients were excluded. The mean patient age at the time of symptom onset was 13.5 years (range, 9−17 years). Nine of 12 patients were female. Eleven of 12 patients had an orthostatic headache as their chief symptom, and 1 patient presented with myelopathy with orthostatic headaches as a secondary symptom. No patients had any major trauma preceding their symptoms. Patient characteristics are summarized in the Online Supplemental Data.
Specific protocols for brain and spine MR imaging varied across institutions. However, all centers generally included similar key sequences for SIH evaluation, including sagittal T1-weighted pre- and postcontrast imaging of the brain, as well as high-resolution 3D imaging of the spine (such as T2 sampling perfection with application-optimized contrasts by using different flip angle evolution [SPACE sequence; Siemens] or CISS/FIESTA sequences). Ten of 12 patients underwent contrast-enhanced brain MRI, demonstrating dural enhancement, brain sagging, and/or venous distention in all 11 patients. One patient underwent a noncontrast MRI of the brain, showing dural thickening and effacement of the suprasellar cistern. Eleven of 12 patients underwent noncontrast MRI of the spine, demonstrating longitudinally extensive (>4 spinal levels) extradural CSF in 7/11 patients (Online Supplemental Data). Five of 7 patients with extradural CSF had a predominantly dorsal CSF collection, 1 of 7 had a diffuse extradural collection (ventral and dorsal), and 1 of 7 had a predominantly ventral collection. Note that the 1 patient who did not have a brain MRI did have a spinal MRI demonstrating extradural CSF, which satisfies the ICHD-3 criteria for a diagnosis of SIH.
All patients underwent some form of advanced myelography, which included prone dynamic CTM (n = 1), decubitus CTM with or without a dynamic component (n = 10), or decubitus DSM with concurrent cone-beam CTM (n = 1).7 Leak types observed included lateral dural tears (n = 7, Figs 1 and 2), CSF fistulas (n = 4, including 3 CVFs and 1 direct CSF-lymphatic fistula, Figs 3–⇓5), and ventral dural tears (n = 1, Fig 6). Five of 7 patients with lateral dural tears had herniating/billowing arachnoid diverticula associated with the dural tear.8 Two of 4 patients with CSF fistulas had a paraspinal venous or lymphatic malformation draining the fistula. The 2 radiologists reviewing the cases concurred on these findings for all patients.
Right T11 (A) and left T4 (B and C) lateral dural tears in a 17-year-old girl and a 12-year-old girl, respectively. In the first patient, a right decubitus photon-counting CT myelogram demonstrates a right T11 lateral dural tear (A, solid arrow) with an arachnoid diverticulum herniating through the dural defect and adjacent epidural contrast (A, dashed arrow). In the second patient, coronal MIP image from a left decubitus CT myelogram shows a left T4 lateral dural tear. An arachnoid diverticulum herniates through the dural defect (B, solid arrow) with epidural contrast accumulating in the lateral epidural space (B, dashed arrows). Intraoperative photograph shows the left T4 lateral dural tear (C, arrow), which was subsequently repaired.
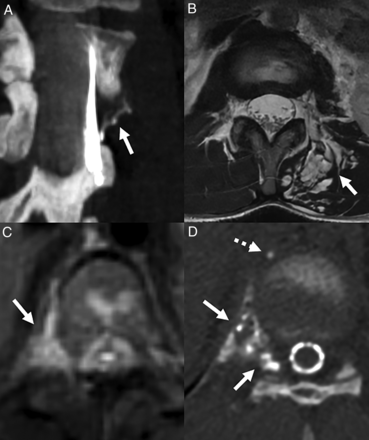
Left T10 lateral dural tear in a 12-year-old girl. Axial 3D T2-weighted image shows a dorsal epidural fluid collection (A, arrows). A left decubitus CT myelogram (B and C) shows a left T10 lateral dural tear with an arachnoid diverticulum herniating through the dural defect (B and C, arrows). Intraoperative photograph shows the lateral dural tear (D, arrow), which was subsequently repaired.
CSF fistulas associated with paraspinal venous and lymphatic malformations in 2 children. In the first patient, a 17-year-old girl with known Klippel-Trenaunay syndrome, a coronal oblique image from a left decubitus cone-beam CTM (A) performed during DSM shows a left L1 CVF (A, solid arrow). The fistula was only very subtly present on the DSM (not shown) and was draining into a lumbar paraspinal venous malformation best seen on T2-weighted MRI (B, arrow). In the second patient, a 9-year-old girl who had a history of kaposiform lymphangiomatosis, axial T2-weighted MRI shows a T2 hyperintense lymphatic malformation (C, arrow). A dynamic CT myelogram shows a right T10 CSF-lymphatic fistula, which drained to a paraspinal lymphatic malformation (D, solid arrows) and the thoracic duct (D, dashed arrow).
A left T6 CVF in a 12-year-old boy with orthostatic headaches. Axial (A) and sagittal (B) reconstructions from a left decubitus CT myelogram demonstrate a CVF (A and B, arrows) draining into the ventral internal epidural venous plexus and left T6 foraminal vein.
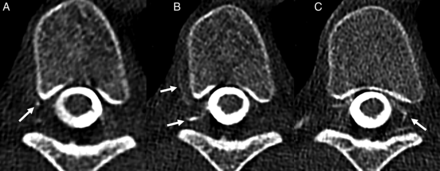
Complex bilateral CVF in a 13-year-old girl with an elevated BMI, presenting with new orthostatic headaches. Selected images from a same-day left (A) and right (B and C) decubitus CT myelograms demonstrate a CVF involving the right T7 foraminal vein (A, arrow), as well as the right T7 internal epidural venous plexus and paraspinal segmental vein (B, arrows). Most interesting, involvement of the left-sided internal epidural venous plexus was also evident on the right decubitus examination (C, arrow). Because it was uncertain from which side the fistula originated, bilateral fibrin glue injection was chosen as the treatment strategy, with follow-up pending.
Ventral dural tear with spinal cord herniation in a 15-year-old girl. Axial (A) and sagittal (B) images from a prone dynamic CT myelogram at the time of presentation show a ventral extradural CSF collection (A and B, dashed arrows). Although the ventral leak was not discreetly seen on these images (which were too delayed to see the ventral leak), the leak site was inferred from a small focus of ventral cord herniation through a dural defect at T5–T6 (A and B, solid arrows). The patient underwent targeted epidural blood patching. Axial (C) and sagittal (D) images from a delayed CT myelogram after treatment demonstrate resolution of the extradural CSF collection, with a small amount of ventral cord adhesion at the prior leak site (C and D, arrows).
All patients eventually underwent targeted treatment of their CSF leak, which entailed a targeted blood patch followed by surgical repair (n = 5), targeted blood and fibrin glue injection (n = 1), targeted blood patch alone (n = 2), targeted fibrin glue injection alone (n = 1), transvenous Onyx (Medtronic) embolization (n = 1), or surgical repair alone (n = 2). Eleven of 12 patients had near-complete or complete symptomatic resolution at the last clinical follow-up, with the mean timing of follow-up being 10.5 months (range, 1−24 months). One patient has not yet had clinical follow-up after the targeted intervention.
Four of 12 patients had underlying genetic or phenotypic disorders (including but not limited to connective tissue disorders) that were thought to be related to SIH, including Marfan syndrome, autosomal dominant lymphedema-distichiasis syndrome (heterozygous FOXC2 mutation), kaposiform lymphangiomatosis, and Klippel-Trenaunay syndrome (Online Supplemental Data).
DISCUSSION
We have described a multi-institutional series of 12 pediatric patients with SIH. In each case, advanced myelography was performed to localize and determine the specific type of leak present. This permitted targeted treatment in all 12 patients, leading to symptom improvement or resolution in 11/12 patients, with 1 patient awaiting clinical follow-up. To our knowledge, this is the first series describing the use of advanced myelography in pediatric patients with SIH. This contributes to the current literature, because myelographic techniques for CSF leak localization have been refined substantially in recent years, particularly since the initial description of CVFs in 2014.9 Despite these advances, most studies on this continuously evolving topic have focused on adults.
Our study allows several insights into SIH in children. First, we found that the typical diagnostic algorithm used for adults with SIH can be successfully applied to children.3 In adults, this entails brain and spine MRI followed by advanced myelography, with positioning for myelography being dictated by the presence or absence of extradural CSF, as well as the distribution of extradural CSF in the axial plane (ventral versus dorsal/lateral).10 Brain MRI findings were abnormal in all 11 patients who underwent the test, and spine MRI reliably helped predict the type of leak present (dural tear in those with extradural CSF, and CVF or CSF-lymphatic fistula in those without extradural CSF).3 Notably, patients with or without extradural CSF can theoretically also have leaks from meningeal diverticula, but these were not observed in our cohort.1,8 For any type of leak, either DSM or CTM (with or without a dynamic component) would be a reasonable option, and this depended on institutional preference in this study. Patients were positioned prone when a ventral dural tear was suspected and positioned decubitus when a lateral dural tear, CVF, or other CSF fistula was anticipated.10,11
Second, we observed that lateral dural tears were the most common type of leak (seen in 7/12 patients), followed by CSF fistulas (4/12). Ventral dural tears were the least common (1/12). Although the use of a multi-institutional, retrospective format limits conclusions about the exact prevalence of different leak types, it is interesting that our findings differ from those in studies on adults, in which ventral dural tears account for a higher proportion of leaks.1,12 Given the association of ventral dural tears with degenerative etiologies such as calcified microspurs, it is probable that the lower prevalence of ventral leaks in the pediatric population reflects the absence of spinal degenerative disease in this age group.13 If this supposition is true, lateral dural tears and CVFs would be expected to be relatively more common in children, and this finding was observed in our cohort.
Third, our study suggests that while connective tissue disorders and/or specific genetic mutations may be present in children with SIH (as seen in 4/12 of our patients), many do not have any identifiable heritable or phenotypic abnormality known to be associated with SIH. In a prior study by Schievink et al,4 connective tissue disorders were more common, present in 13/24 patients. The lower prevalence of these and other disorders in our cohort could be secondary to underdiagnosis or selection bias. Nonetheless, both studies suggest that heritable disorders are not universally present in children with SIH, and the diagnosis should be considered even in patients without these syndromes.
Fourth, it is interesting that 2/4 patients with CSF fistulas (CVFs or CSF-lymphatic fistulas) had paraspinal venous or lymphatic malformations. Several prior studies have shown that CVFs can be associated with such vascular lesions, and this finding was particularly common in our pediatric cohort.14⇓⇓-17 These lesions may be especially important in children with SIH, and we encourage close scrutiny of premyelographic MRI or other imaging to search for such paraspinal abnormalities.
When advanced myelography is pursued to localize CSF leaks in children, care should be taken to minimize the radiation dose. DSM generally has a lower radiation dose than CTM, though availability and experience with DSM vary across institutions.18 While DSM has better spatial and temporal resolution, only CTM provides cross-sectional detail, which is helpful and sometimes necessary to localize leaks. CTM also provides better contrast resolution, which may be helpful in detecting particularly subtle leaks.19 The dose for CTM (especially dynamic CTM) can be mitigated by reducing the number of scans performed and limiting the scan range to the area of interest, which are the main factors that can cause dynamic CTM or other techniques to have a higher dose than conventional CTM.20,21 Examples of applying this type of dose reduction would include scanning only levels where extradural CSF is present or focusing the search for a CVF to levels with paraspinal venous malformations. Photon-counting detector CT generally has a lower radiation dose than energy-integrating detector scanners, representing another way to reduce the dose in these patients.22,23 Intrathecal gadolinium MR myelography was not used in any of our patients, largely because it is generally considered to have a low diagnostic yield. Nonetheless, this would be a theoretic option to avoid ionizing radiation altogether.24,25
Regarding procedural techniques, needle size and type and contrast dose are important considerations for any type of myelography. Many radiologists advocate the use of small (22−25 ga) atraumatic needles to reduce the risk of post-lumbar puncture CSF leaks.26 These may be particularly important in children with connective tissue disorders, whose dura may take longer to heal. The intrathecal contrast dose is another important consideration, and specific guidelines and evidence for pediatric patients are lacking. Ultimately, there remains substantial variability among institutions in this regard, and further study of this topic is needed. In our practices, patient and proceduralist preference usually dictates the type of needle used. Many factors such as the patient’s weight, the presence or absence of meningeal diverticula, and the type of myelography being performed dictate the total contrast dose and concentration used.
Our study has limitations, one of which is its relatively small sample size. Despite reviewing databases from 5 institutions during a several-year period, we identified only 12 children with SIH who underwent advanced myelography. SIH has an overall incidence of approximately 4−5 per 100,000 per year in population-based studies.27,28 The incidence in children is likely lower, and because only a subset of children with SIH undergoes advanced myelography, relatively few patients met our stringent inclusion criteria. Additionally, we recognize that not all institutions routinely perform advanced myelography in patients with SIH, in part because some can be cured with nontargeted blood patching or conservative measures. This approach is reasonable for some patients, and ultimately many factors such as patient and provider preference and institutional resources must be weighed to guide work-up and treatment strategies. Many types of treatment, including Onyx embolization, targeted fibrin injection, and surgery necessitate leak localization.29,30
CONCLUSIONS
DSM and specialized variations of CTM to localize dural tears and CSF fistulas can be successfully applied to children with SIH. Many interesting differences in leak types and frequency compared with adults were apparent in our pediatric cohort, and future studies will be needed to determine whether this finding represents selection bias versus true variation between children and adults.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received June 10, 2024.
- Accepted after revision July 8, 2024.
- © 2024 by American Journal of Neuroradiology