SUMMARY:
Four distinct vascular anomalies can be seen to affect the brain on fetal imaging: vein of Galen malformations, nongalenic arteriovenous pial fistulas, dural sinus malformations, and intracranial venous malformations. These congenital disorders affect the arteries and veins of the developing brain and are rarely seen beyond the neonatal stage. The 4 fetal cerebrovascular anomalies are associated with quite disparate natural histories and prognoses. MRI plays a pivotal role in the evaluation of fetuses with these conditions because of its ability to definitively establish the diagnosis, to detect subtle parenchymal injuries, to delineate the course of abnormal vessels in detail and to some extent the nature of vascular flow, and to identify ischemic, thrombotic, and hemorrhagic complications. Recently, an investigational transuterine embolization procedure targeted at treating fetuses with vein of Galen malformations who are at high risk for neonatal decompensation has emerged as a promising alternative to expectant management and postnatal embolization, with imaging being used to identify suitable patients for the intervention and in preprocedural planning. This manuscript reviews the essential imaging and clinical features of these 4 fetal neurovascular anomalies and underscores the practical aspects related to counseling, prognosis, and the multidisciplinary management of these entities.
ABBREVIATIONS:
- b-SSFP
- balanced steady-state free precession
- DSM
- dural sinus malformation
- icVM
- intracranial venous malformation
- RASA1
- RAS P21 protein activator 1
- SSFSE
- single-shot fast spin-echo
- VOGM
- vein of Galen malformation
Historically, congenital cerebral vascular anomalies were diagnosed in symptomatic neonates postpartum.1,2 The improvements in fetal ultrasonography and MRI now allow for the detection and characterization of these anomalies prenatally. Despite the low incidence of these conditions in the general population, these disorders are collectively encountered with relative frequency in subspecialized maternal-fetal care centers and children’s hospitals.3,4 To interpret these diagnostic studies accurately, radiologists must be familiar with the imaging characteristics, natural progression, and clinical trajectory of this distinctive group of disorders.
In this manuscript, we review the key imaging and clinical features of 4 congenital cerebrovascular lesions: vein of Galen malformation (VOGM), nongalenic pial AVFs, dural sinus malformations (DSMs), and intracranial venous malformations (icVMs). We will emphasize the role of fetal MRI in the diagnosis and triage of these conditions, covering all aspects of image acquisition and interpretation. Furthermore, we will highlight the similarities and differences among these disorders and frame the discussion around practical implications pertinent to counseling and multidisciplinary management of the respective entities. Rarely, other vascular lesions that typically occur in late childhood and adulthood, such as cerebral cavernous malformations, have been reported in fetal life.5 These lesions will not be covered in detail in this manuscript, as their fetal onset constitutes an exception to their usual epidemiology, and their biology and appearance are distinct from the 4 unique lesions described above.
IMAGING
Most fetal cerebrovascular anomalies are diagnosed during the routine second-trimester fetal sonography.6 Fetal sonography not only allows for early detection of these malformations but also allows for the identification of vascular flow by utilizing color Doppler analysis.7⇓-9 Invariably, a fetal MRI should be obtained in cases where a neurovascular anomaly is suspected to confirm the diagnoses, characterize the anatomy, and evaluate for any parenchymal injuries.10,11
MRI is the preferred imaging technique to evaluate fetuses suspected of having intracranial vascular lesions. Its high-quality soft tissue contrast offers insights into the brain parenchyma, and its multiplanar capabilities allow for the precise localization of abnormalities. Moreover, the large field of view images provide information about the entire fetus, which sheds light on possible systemic hemodynamic repercussions of the intracranial abnormalities. Fetal MRI is performed without sedation or intravenous contrast, and the images can be acquired by using either 1.5 or 3T magnets. In-depth knowledge of these vascular disease processes and their appearance on MRI allows the interpreting physician to assess fetal neuroanatomy and flow dynamics and comment on potential ischemic, hemorrhagic, or thrombotic complications.12 The Table summarizes the fetal MRI protocol utilized at the authors’ institution for the evaluation of these entities.
MRI protocol for evaluation of fetal cerebrovascular anomalies
Neuroanatomic images are acquired with rapid MRI techniques. T2-weighted single-shot fast spin-echo (SSFSE) sequences use a long echo-train length to efficiently sample k-space and encode 1 slice per repetition time. Due to its spin-echo acquisition, flow within large vessels manifests as a signal void.12,13 Anatomic images are also acquired with a balanced steady-state free precession (b-SSFP) sequence. In this rapid gradient-echo technique, flowing blood appears hyperintense. This sequence has a high SNR, beneficial for delineating small structures and mitigating artifacts (eg, dielectric effect); however, tissue characterization is somewhat limited by its mixed T1/T2 contrast properties.12,13 These 2 sequences constitute the workhorse for the anatomic evaluation of normal and abnormal vascular structures, the parenchyma, and the CSF spaces.
DWI is a crucial component of fetal neurovascular protocols. Most centers acquire fetal DWI with b-values of b = 0 and b = 700 to preserve SNR. Institutions may opt to apply diffusion encoding gradients in 3–12 noncollinear directions, balancing acquisition speed and SNR. Diffusion-weighted MR imaging allows for the detection of acute ischemic injury and organized clots.14,15 T2*-weighted sequences rely on an echo-planar imaging acquisition with a long time-to-echo to accentuate transverse magnetization decay and are helpful for detecting blood products and venous congestion.16
A rapid gradient-echo T1-weighted sequence can cover the entire uterus in a single breath-hold. Evaluating the parenchyma for foci of T1 shortening can pinpoint areas of subacute injury that may be unapparent on T2 SSFSE or b-SSFP. T1 hyperintense signal may also reveal subacute blood products within a clot.12 Furthermore, due to the gradient-echo acquisition with a short repetition time, the sequence is sensitive to flow effects, aiding in the detection of fast-flow within vascular structures.4
More recently, Sampaio and colleagues17 have refined a 2D time-of-flight technique, another gradient-echo acquisition variant, to highlight flow-related signals within fetal vessels. The results of this preliminary study are promising, with consistent identification of the torcular herophili, vein of Galen, internal cerebral veins, basilar artery, and middle cerebral artery. However, due to the relatively long acquisition time, exceeding 3 minutes, the sequences had substantial artifacts in over 70% of cases.17 It is likely that with further developments in image acceleration techniques and with the extension of this work to 3T MRI, the results will be enhanced, thus enabling reliable identification of these and other major vascular structures.
FETAL CEREBROVASCULAR ANOMALIES
Vein of Galen Malformation
A VOGM, while constituting less than 1% of all intracranial vascular malformations, is the most commonly seen congenital brain vascular anomaly, occurring in approximately 1:58,100 deliveries18 and makes up nearly one-third of intracranial vascular anomalies in children.19,20 These malformations develop between the 6th and 11th weeks of gestation during cerebral angiogenesis.6 Recent genetic analysis has identified mutations in RASA1, Ephrin type-B receptor 4, activin A receptor like type 1, neurogenic locus notch homolog protein 1, integrin subunit beta 1, and protein tyrosine phosphatase non-receptor type 11 in VOGM probands, with endothelial cells localized spatiotemporally at the affected cellular locus.21 VOGMs result from pathologic fistulous connections between the choroidal arteries and the median prosencephalic vein, resulting in a high-flow arteriovenous lesion that induces arterialization of flow in the median prosencephalic vein with progressive dilation.3,10 Notably, the term “malformation of the vein of Galen” is a misnomer, as ontologically, the anomaly precedes the establishment of a mature vein of Galen, and the locus of the malformation is in the anterior aspect of the prosencephalic vein, which normally involutes entirely.6
Imaging.
Despite their embryologic origin in early gestation, VOGMs are rarely detected before the second trimester’s detailed anatomic survey and, occasionally, are identified even later.22,23 Sonographically, the malformation presents as a midline hypoechoic lesion within the quadrigeminal cistern. Color Doppler imaging reveals rapid and turbulent flow within the varix and may, occasionally, identify the feeding arteries.9
On fetal MRI, the VOGM appears as a dilated varix located in the quadrigeminal cistern, just dorsal to the tectal plate (Fig 1). On T2-weighted SSFSE images, the varix presents as a large flow void, while on b-SSFP images, it exhibits as a hyperintense signal due to the flow within the lesion.24 Occasionally, the high flow within the varix may appear as a hyperintense signal on T1-weighted gradient-echo images. Lasjaunias25 classified VOGMs based on the angioarchitecture of the arterial feeders into the choroidal type, characterized by numerous feeder vessels, and the mural type, which has a smaller number of large-caliber, fistulous feeders. This distinction can be apparent on T2-weighted SSFSE, leveraging the inherent contrast between the flow voids and the CSF, though most VOGMs do not neatly fit into this bifurcated paradigm, as they contain a complex inflow arterial network, within which 1 or 2 large pedicles are fistulous. The venous drainage of the varix is also distinctly outlined on imaging. The most common drainage pattern is via a persistent falcine sinus, but drainage via a straight sinus or a combination of the two is seen as well.26
Vein of Galen malformation in a 35-week-old fetus. SSFSE in the (A) coronal, (B) axial, and (C) sagittal planes shows a varix in the quadrigeminal cistern corresponding to the dilated median prosencephalic vein. No parenchymal injury was identified.
The fetal MRI examination also serves as a survey for nonvascular findings. Though uncommon, parenchymal injuries may occur because of progressive venous hypertension (Fig 2). DWI can reveal areas of decreased diffusivity in the deep white matter indicative of venous ischemia.27 Additionally, zones of venous congestion and hemorrhage may become apparent as areas of low signal intensity on T2*-weighted sequences (Fig 3). As injury progresses, cystic encephalomalacia may develop. In the late subacute or chronic phases, injuries from these causes might present as nonspecific areas of T1 hyperintense or T2 hypointense signal. Mild ventriculomegaly is often observed in fetuses with VOGM, likely stemming from elevated venous pressure, which impedes CSF absorption into the venous system. If the ventriculomegaly is moderate to severe or asymmetrical, the possibility of ex vacuo dilation due to tissue loss should be contemplated.27
Vein of Galen malformation with parenchymal injuries in a 35-week-old fetus. A, Axial T2 SSFSE shows a dilated median prosencephalic varix, falcine sinus, and torcular, as well as ventriculomegaly. B, Axial T2 SSFSE shows areas of cystic degeneration (black arrow) and T2 hyperintensity in the left frontal lobe (white arrow), representing venous ischemia. C, Neonatal coronal T2 image demonstrates progression of parenchymal involvement, with new areas of ischemic injury in the right frontal lobe and insula (white arrow) associated with moderate ventriculomegaly.
Parenchymal injuries in 2 fetuses with vein of Galen malformations. A, Axial DWI in a 31-week-old fetus shows restricted diffusion (white arrow) in the left temporal lobe. B, Coronal T2 SSFSE shows generalized volume loss, cystic change (black arrow), and T2 hyperintense signal (white arrows) related to encephalomalacia. C, Axial T2* echo-planar image in a different 24-week-old fetus shows hemorrhage in the left periventricular region.
Natural History.
The landscape surrounding the counseling and management of fetuses with VOGMs is evolving rapidly because of the ability to confidently prognosticate clinical presentation based on fetal imaging as well as to the advent of a prenatal treatment option.3 Until recently, management in utero was largely expectant, as only postnatal endovascular procedures were available.28,29 Reviewing the natural history of VOGMs, including the outcomes of patients undergoing postnatal embolization, is helpful in understanding the drive toward new therapeutic avenues and the role of imaging.
The presence of a VOGM markedly alters fetal hemodynamics by creating a large arteriovenous shunt that precipitates a state of high cardiac output with systemic repercussions.30 Echocardiographic indicators of this hyperdynamic state include severe dilation of the superior vena cava, an increased cardiothoracic ratio, reversal of diastolic flow in the transverse aortic arch and sometimes the descending aorta, and right ventricular dysfunction.31 Fetal MRI may further illustrate high-output features such as cardiomegaly and subcutaneous tissue edema in the head, neck, and extremities.24 Despite pronounced hemodynamic compromise, overt cardiac failure or cardiovascular collapse is rarely encountered prenatally, likely because of the low-resistance placental circulation’s protective compensatory mechanisms.
Neuroprognostication for individuals prenatally diagnosed with VOGM is challenging.19 The rarity of this condition and variability in medical care, including the impact of pregnancy terminations and care redirection during the neonatal period, confound the reporting of long-term outcomes for fetuses newly diagnosed with VOGM. In a study spanning multiple institutions, which included 49 fetuses with VOGM, Paladini and colleagues32 reported a 37% survival rate free of neurologic complications within the overall cohort. This figure rose to 53% when excluding subjects who had undergone selective termination.32 More recently, a meta-analysis of all published fetal VOGM series from D’Amico et al30 demonstrated a likelihood of 35.4% of survival past infancy free of neurologic impairment for all fetuses diagnosed with VOGM.
Patients can be more precisely categorized based on their neonatal presentation. Broadly, cases cluster into 3 distinct groups. A minority, ranging from 10%–18%, demonstrate extensive brain injuries on a neonatal brain MRI or progression of prenatally diagnosed brain injuries; these neonates are generally not considered candidates for embolization.32,33 A second group, comprising nearly 60% of cases, present with severe hemodynamic instability within the first 72 hours but have no substantive brain injuries. Termed “neonates at risk” by clinicians and researchers, these patients often derive benefit from prompt embolization. However, the prospects for survival with favorable neurologic outcomes are variable, ranging from 10% to 48%.29,33 A third group consists of neonates without brain injury and with minimal hemodynamic compromise. For these infants, embolization can be deferred until later in infancy, and they generally have a better neurologic prognosis. Known as the “infantile treatment cohort” or “postneonatal treatment cohort,” these patients experience positive neurologic outcomes in 58%–77% of cases.3,29,33
Imaging and Prenatal Risk Stratification.
The previous review of clinical trajectories for neonates with VOGM underscores the vulnerability of the “neonates at risk” group, which represents the largest cohort of fetal diagnosed VOGM.33 Although emergent postnatal embolization can be lifesaving for these neonates, it may not prevent neurologic disability.30 Prenatal identification of these “at-risk” patients could open a critical window of opportunity to prevent circulatory collapse in the neonatal period and its neurologic sequelae. However, this pursuit necessitates reliable fetal biomarkers that differentiate these patients from fetuses with established brain injury,27 who would not benefit from fetal intervention, and from those who are likely to have a mild neonatal course, allowing embolization to be safely deferred until infancy.
Jaimes and colleagues27 investigated the significance of brain injury on fetal MRI. Their series showed that all forms of brain injury, including restricted diffusion, localized gliosis, volume loss, or venous patterns of injury, invariably progressed. This indicates that fetal MRI can reliably identify fetuses with end-organ injury to the brain who are unlikely to benefit from intervention. On the other hand, Arko and colleagues34 sought to find a fetal biomarker predictive of neonatal cardiopulmonary decompensation. They found that the point of greatest constriction of the drainage pathway of the malformation, typically the falcine sinus, reliably forecasted the necessity for neonatal intervention, likely because of its function as a flow-limiting anatomic barrier. Specifically, a smaller mediolateral diameter or cross-sectional area of the falcine or straight sinus at its narrowest point correlated with a lower risk. Among these metrics, the mediolateral diameter is preferable for clinical use because of its ease of measurement. The risk of neonatal decompensation for subjects with a mediolateral diameter of 2 mm and 3 mm was 14% and 26%, respectively (Fig 4). In contrast, the risk for diameters of 9 mm and 10 mm was 93% and 96%, respectively.34
Measurement of the mediolateral width of the falcine sinus for consideration of fetal intervention and postintervention imaging. A, Axial T2 image at 32 weeks soon after the initial diagnosis of VOGM, with intact parenchyma and mild ventriculomegaly. B, Axial T2 image at 34 weeks, just before fetal embolization, shows interval growth in sinus width. C and D, Coronal T2 images prefetal embolization and 1-day post embolization respectively, showing diminution in width of the falcine sinus, indicative of diminution in lesional flow.
Treatment (Fetal Embolization of VOGM).
Over the past 2 decades, a considerable amount of evidence has accumulated demonstrating the clinical effectiveness of various fetal interventions and the technical proficiency required to implement fetal therapy programs both safely and effectively. Reflecting this advancement, a multidisciplinary team at Boston Children’s Hospital has launched a prospective clinical trial to evaluate the efficacy of prenatal embolization in fetuses diagnosed with VOGM who are at an elevated risk of neonatal decompensation.18 The criteria for participant inclusion and exclusion in the trial are detailed in the Online Supplemental Data.
The trial18 consists of a single embolization procedure performed via a percutaneous maternal approach with sonography guidance. Using catheters, wires, and coils identical to those used in postnatal embolizations, a team of maternal-fetal specialists, radiologists specialized in fetal imaging, and neurointerventionalists employ fetal sonography for real-time imaging and color Doppler to monitor blood flow. By relying on sonography guidance, the need for ionizing radiation is entirely circumvented. The procedure entails the transuterine insertion of an 18 G needle, guided transcranially through the occipital bone into the fetal torcular. Following this, a microcatheter and microwire are navigated to the prosencephalic varix, where embolization is performed with platinum detachable coils. The embolization aims for a packing attenuation of 15%–20%, as determined by fetal MRI measurements, to substantially reduce blood flow through the malformation while avoiding complete occlusion of the varix (Fig 4).
While the trial is still in its early stages,18 Orbach and colleagues35 published a case report detailing the successful transuterine embolizations of a third-trimester fetus with a VOGM. The patient selected for the procedure was a 34-week fetus with a malformation that predicted a high risk of neonatal distress due to a falcine sinus width greater than 10 mm. Following fetal intervention, the cardiac output of the fetus decreased by 40%, and the transverse diameter of the falcine sinus reduced from 13 to 8 mm on fetal imaging.35 More notably, after birth the baby required no neonatal embolization and no cardiopulmonary support with intubation or medication; neurologic status has been normal (the patient is now 11 months old).
The postnatal management of VOGM is complex and beyond the scope of this review, which focuses on the fetal care of these patients. As mentioned above, hemodynamic stability in the first few days after birth dictates the need for early embolization. Meanwhile, sustained hemodynamic stability offers the advantageous option of delaying embolization until infancy, allowing the brain to mature and potentially improving outcomes. The manuscripts by Brinjikji et al29 and Yan et al36 cover these topics in detail.
Nongalenic Pial Fistulas
Less commonly, anomalous arteriovenous communications may occur between vessels located along the pial surface of the brain. These pial AVFs are also referred to as nongalenic fistulas, emphasizing that they arise from an embryologic pathway different from VOGMs and, therefore, can occur in almost any intracranial location along the surface of the brain.37
Imaging.
Imaging reveals pial AVFs as dilated, tortuous vascular structures along the brain’s surface. Frequently, a single enlarged draining vein is noted without an appreciable nidus.38 Predictably, these vessels exhibit robust flow voids on SSFSE sequences, intermediate to high signals on b-SSFP imaging, and flow-related signals on gradient-echo T1-weighted images. A notable characteristic of pial AVFs is their off-midline position (Fig 5). When the shunt is situated over the hemispheric convexities, differentiation from a VOGM is straightforward. Nevertheless, pial AVFs may occasionally be found in the deep interhemispheric region or close to the midline in the choroidal fissure and be somewhat reminiscent of a VOGM.39 The identification of asymmetrical or eccentric drainage veins that do not conform to the classic configuration of a median prosencephalic venous varix can serve to differentiate these 2 entities.
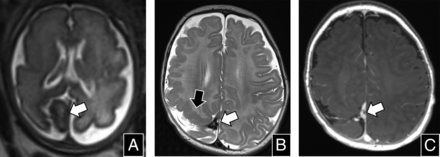
Pial arteriovenous fistula in a 32-week-old fetus. A, Sagittal T2 SSFSE shows a flow void in the frontal interhemispheric region (arrow) that drains into the deep venous system and eventually into the torcular. B, Sagittal T2 SSFSE through the right hemisphere shows the prominent pial vessels (white arrows), congestion of medullary veins, and developing periventricular cystic change. C, Coronal T2 SSFSE shows right greater than left parenchymal cystic changes (arrow), related to encephalomalacia and associated mild ventriculomegaly. D, Coronal T2* echo-planar image obtained 2 weeks later shows areas of blooming in the periventricular regions that correspond to areas of T1 shortening on (E) coronal T1 gradient-echo imaging (white arrows in D and E, respectively), related to venous congestion and subacute blood products.
Natural History.
The clinical course of pial AVFs diagnosed in utero is quite variable. Many of these present with signs of high-output cardiac failure prenatally, which may include cardiomegaly and hydrops.39 Prenatal fetal MRI may reveal areas of brain injury, such as restricted diffusion, gliosis, or hemorrhage. Although based on limited observations, these injuries tend to be asymmetrical and localized to the territory involved in the fistula; this is in contrast to the more diffuse and symmetric pattern of brain injury observed in fetuses with VOGM.40 In severe instances, brain injury may become extensive; however, a gradient of severity toward the fistula site is generally discernible. Similar to VOGM, the presence of prenatal brain injury is a dire indicator, suggesting a progressive cascade of ischemic injuries caused by venous hypertension. However, unlike for VOGM, in some cases of pial AVF, even extensive parenchymal loss adjacent to the AVF site may not be accompanied by clinical signs and symptoms, with patients demonstrating remarkable neuronal plasticity and functional remapping.38
Treatment.
Currently, there are no prenatal alternatives for the treatment of (nongalenic) pial AVFs. Their variable location across the brain does not lend itself well to the structured access pathway that has been devised for fetal embolization of VOGM. Pial AVFs have been associated with mutations in HHT genes and in RASA141 and, invariably, these lesions require postnatal embolization, as they are high-flow shunting lesions that pose a high risk of growth and intracranial hemorrhage.42
Dural Sinus Malformations
DSMs are rare intracranial congenital vascular anomalies; their precise incidence is unknown but estimated to be very low, with less than 80 published cases in the literature before 2018.43 This entity was first described in the neurointerventional literature as low-flow dural arteriovenous fistulas, presenting in neonates and infants with enlarged venous pools within the affected dural sinus, often at the torcular.4,43 With advancements in prenatal sonography and MRI, DSMs can now be identified before birth. A comprehensive understanding of the pathophysiology and prognosis of in utero-diagnosed DSMs is essential for appropriate counseling and management of these patients.
Imaging.
Most DSMs are diagnosed during the mid-second trimester sonographic survey. Often, ultrasound can readily identify a hypoechoic and dilated venous structure at the location of the involved dural sinus.44 Interrogation with color Doppler is crucial to confirm the vascular nature of the abnormality and to differentiate it from cystic abnormalities. An intraluminal clot may occasionally pose a diagnostic challenge, as the clot may show varying degrees of echogenicity, and demonstrating flow in a narrowed lumen can be difficult or impossible. MRI is invariably indicated in the setting of a suspected DSM to confirm the diagnosis and to assess the integrity of the brain parenchyma.4
Fetal MRI provides exquisite delineation of vascular and parenchymal anatomy. On T2-SSFSE images, the dilated dural sinus—often the torcular—appears as an enlarged tubular or saccular flow void (if patent) (Fig 6). On b-SSFP images, the DSM is relatively hyperintense due to the effects of flowing blood. The lumen of the dilated sinus and other intracranial venous structures must be carefully inspected for patency. A clot may appear as T1 hyperintense material with diffusion restriction within the DSM or other sinuses.45 Signal alterations on T2 SSFSE (loss of flow void) or b-SSFP images, although more subtle, may also indicate thrombus. T2* sequences serve as valuable adjuncts to identify clots; however, findings should be corroborated on other sequences due to the inherently black-blood background (Fig 7). Finally, the T1 gradient-echo images (or a dedicated flow-sensitive sequences) should be carefully inspected for evidence of arterialized flow into the malformation, which appears to bear prognostic significance.4 The arteries most commonly involved in DSMs are branches of the middle meningeal artery, occipital artery, and posterior auricular artery.4
Dural sinus malformation in a 33-week-old fetus. A, Sagittal T2 SSFSE shows marked dilation of the posterior aspect of the superior sagittal sinus and torcular. B, Sagittal DWI confirms the presence of clot within the dilated sinus (arrow). The brain parenchyma appears normal.
Dural sinus malformation in a 32-week-old fetus who was referred with a diagnosis of ventriculomegaly. A, Fetal sonography revealed a dilated dural sinus with internal echogenicity representing clot (black arrow). The echogenicity of the parenchyma was also abnormal (white arrow), with cystic changes and volume loss associated with severe ventriculomegaly. B, Axial T2 SSFSE shows severe parenchymal injury with cystic encephalomalacia (white arrows), severe ex vacuo ventriculomegaly, and intermediate signal from clot within the distended torcular (black arrow). C, Axial T1 gradient-echo shows blood products within the torcular that demonstrate T1 hyperintensity (black arrow).
Natural History.
It is imperative to recognize a discordance between the evolution and reported outcomes of DSMs diagnosed prenatally and postnatally. Collective accounts by various investigators, including Yang et al4,43 and Merzoug et al,46 indicate survival of fetuses affected by DSMs without significant neurologic sequelae in 83% and 88% of cases, respectively. These statistics contrast markedly with the classic neuroangiographic reports of DSMs diagnosed in neonates and infants, such as those detailed by Barbosa et al,47 which presented a grim prognosis with severe neurologic disability or death in 46% of subjects. This discordance is likely due to a combination of ascertainment bias and improvement in management over the past 2 decades. Neonatal and infantile DSMs reported in the neuroangiography literature likely represented a sampling of noninvoluting malformations with unfavorable physiology, while spontaneously involuting fetal and asymptomatic neonatal DSMs likely remained undiagnosed.4,43
Given the relatively low number of reported cases, describing the natural history of prenatally diagnosed DSMs remains challenging. Intraluminal thrombosis is observed almost invariably in fetal DSMs, probably due to the slow flow within the distended venous lake. In the systematic review published by Yang and colleagues,43 97% of the DSMs exhibited a spontaneous decrease in size, with a single fetus experiencing a transient increase followed by subsequent shrinkage. Although difficult to detect, arterialized flow was reported by Yang and colleagues4 in approximately one-third of cases (Fig 8). In their analysis, the presence of a clot and a shrinking DSM were identified as favorable prognostic factors, whereas parenchymal injury, ventriculomegaly, and the persistence of arterialized flow were associated with an adverse prognosis.4,43
Dural sinus malformation in a 33-week-old fetus. A, Fetal sonography with color Doppler shows turbulent flow within a dilated torcular. B, Axial T2 SSFSE shows an enlarged flow void in the region of the torcular, without signs of clot. There were no sonographic changes in the brain parenchyma. C, Axial T1 gradient-echo shows flow-related signal within enlarged external carotid arteries bilaterally (arrows). D, 3D maximum intensity projection from the postnatal time-of-flight MRA shows arterialized flow within the dural sinus malformations and middle meningeal and occipital artery feeders (arrows).
Treatment.
Currently, there are no prenatal treatment options available for fetal DSMs, and given the prevailing notion that these entities have a favorable prognosis, such treatments are unlikely to be required. When a DSM is diagnosed in the early second trimester, it is prudent to obtain a follow-up in the third trimester to evaluate the trajectory of the DSM and the clot and to perform a query for arterialized flow. In all cases, a postnatal MRI should be obtained to further evaluate the anatomy and definitively exclude arterialized flow.
Intracranial Venous Malformations
An icVM is a recently identified entity that does not conform to a disease category recognized in the 2018 classification of the International Society for the Study of Vascular Anomalies (ISSVA) in 2018.48 This framework recognizes brain arteriovenous malformations, arteriovenous fistulas, and cerebral cavernous malformations but does not recognize an intracranial entity homologous to venous malformations elsewhere in the body. Chen et al49 documented the imaging characteristics of icVMs that constituted approximately 2% of the total cohort of patients with cerebrovascular anomalies reviewed in their tertiary academic referral center and that mirror those of well-known extracranial venous malformations.50 Their research49 includes a series of fetal and postnatal pediatric cases, describing these malformations as well-defined lobulated lesions in continuity with either the superficial or deep venous system of the brain. These differed from both the caput medusae configuration of developmental venous anomalies and from sinus pericranii lesions, as they do not span the cranial vault. In cases with postnatal presentations, the enhancement patterns on conventional angiography or postcontrast MRI matched those of other intracranial venous structures.49
Imaging.
On ultrasound, icVMs appear as well-circumscribed, extra-axial, lobulated lesions, frequently in a parafalcine or paramedian location, without arterialized flow on Doppler. Their appearance is nonspecific, and a final diagnosis is unattainable without additional imaging. On MRI, the lesions present as well-defined T2 hypointense structures, showing no indications of arteriovenous shunting, such as dilation of collecting veins or pulsatility (pulsation artifacts) (Fig 9). Chen and colleagues49 identified 3 morphologic subtypes of icVMs: globoid, fusiform, and tubular. In their series, among the 3 cases diagnosed during fetal life, 2 were falcine, and 1 was globoid.
Intracranial VM in a 31-week-old fetus (white arrows in A to C). A, Axial T2 SSFSE shows a globoid soft tissue structure with internal signal heterogeneity located in the right posterior parafalcine region (arrow); the subjacent parietal parenchyma appears abnormally folded and hypointense. B, Axial T2 and (C) postcontrast axial MPRAGE demonstrate partial involution of the structure since the fetal examination (white arrows in B and C), with polymicrogyria adjacent to the lesion (black arrow in B). Persistent enhancement and a somewhat tubular and branching architecture are apparent on postcontrast imaging (C).
Natural History.
The icVMs were associated with complex extracranial venous malformations in 79% of cases and with sinus pericranii in nearly 50% of cases. Consequently, fetal MRIs should be thoroughly evaluated for these potential associations. Even when such findings are not evident on prenatal imaging, postnatal MRI is advisable to fully delineate the anatomy and exclude associated anomalies. Although longitudinal follow-up data are scarce given the recent identification of these lesions, icVMs appear to remain stable over time and may be clinically silent.49
Treatment.
Because icVMs were only recently identified and the current evidence indicates that they are clinically silent in fetal life, there appears to be no reason to develop prenatal treatment approaches.
TAKE HOME POINTS
In fetuses with VOGM, it is critical to recognize prognostic indicators. The presence of parenchymal injury carries a poor prognosis, as it often signifies the initiation of a venous ischemic cascade. The mediolateral (transverse) diameter of the falcine sinus should be estimated because larger dimensions predict a higher risk of neonatal hemodynamic collapse with the need for urgent embolization.
A vascular lesion with arterial inflow, located anywhere other than the characteristic location of VOGMs, is indicative of a pial AVF. These malformations exhibit a variable prognostic range. Pial AVFs may lead to localized areas of parenchymal injury or high-output cardiac failure.
A DSM should be considered in any fetus presenting with a dilated dural sinus, regardless of arterial inflow or the presence of a luminal clot. Most patients exhibit a favorable prognosis, independent of the size of the DSM. Shrinkage of the DSM during fetal life is reassuring. Conversely, the presence of arterialized flow may necessitate closer postnatal follow-up.
A lobulated or “fleshy” lesion without arterial inflow should raise the possibility of an icVM, especially if parasagittal or parafalcine. As with most venous malformations in the body, the prognosis is excellent.
CONCLUSIONS
Fetal cerebrovascular anomalies represent a group of 4 conditions with diverse clinical outcomes and prognoses. Prenatal imaging, especially fetal MRI, is vital for the diagnosis and detailed characterization of these lesions. This is increasingly important with the advent of prenatal therapeutic options for certain conditions, such as VOGM. Familiarity with the imaging features, natural history, and potential outcomes of these rare disorders is essential for the accurate interpretation of these complex studies.
Footnotes
This work was supported by the American Roentgen Ray Society Scholarship; Career Development award from the Office of Faculty Development at Boston Children’s Hospital; National Institute of Neurological Disorders and Stroke, grant/award Nos. R01EB031849, R01EB032366, R01HD109395, R01NS106030; National Institutes of Health Office of the Director, grant/award No. S10OD0250111; Rosamund Stone Zander Translational Neuroscience Center, Boston Children’s Hospital; National Institute of Biomedical Imaging and Bioengineering; and Eunice Kennedy Shriver National Institute of Child Health.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received March 3, 2024.
- Accepted after revision June 1, 2024.
- © 2025 by American Journal of Neuroradiology