Abstract
BACKGROUND AND PURPOSE: Parasagittal and superior sagittal sinus (SSS) dural arteriovenous fistulas (DAVFs) are often inappropriately classified. We explore the clinical presentations, imaging characteristics, and endovascular treatment strategies of these 2 DAVF subtypes.
MATERIALS AND METHODS: Clinical and imaging data of 19 patients with SSS or parasagittal sinus DAVFs who underwent endovascular treatment in our institution between 2017 and 2022 were retrospectively analyzed. The angiographic findings, endovascular treatment strategies, and angiographic outcomes were evaluated and recorded.
RESULTS: Among these 19 patients, 14 had a parasagittal DAVF, 4 had a SSS DAVF, and 1 patient had both parasagittal and SSS DAVF. Only 1 (1/19, 5.26%) patient presented with intracranial hemorrhage. For the parasagittal DAVF group, most of the shunts were located along the middle third of the SSS (12/15, 80%), on the dura in proximity with the junctional zone between the bridging vein and SSS (15/15, 100%), with ipsilateral cortical venous reflux (CVR) (15/15, 100%). For the SSS DAVF group, all 5 patients had shunting zone along the middle third of the SSS, on the sinus or parasinus wall, with bilateral CVR. Transarterial embolization, via the middle meningeal artery as the primary route of access, was the primary treatment approach in 95% of cases (19/20). Reflux of embolization material into the SSS was observed in 1 case (1/5, 20%) of SSS DAVF in which balloon sinus protection was not used during embolization.
CONCLUSIONS: Our study found that parasagittal DAVFs have shunting point(s) centered on the junctional zone of the bridging vein and the SSS with ipsilateral CVR, while SSS DAVFs have shunting point(s) centered on the sinus or parasinus wall with bilateral CVR. Transarterial embolization via the middle meningeal artery can be used as the primary treatment strategy in most cases. Balloon sinus protection during embolization is not necessary in cases of parasagittal DAVF with occluded or stenosed connection with the SSS but its use should be considered in cases of SSS DAVF with patent sinus.
ABBREVIATIONS:
- CVR
- cortical venous reflux
- CFD
- computational fluid dynamics
- DAVF
- dural arteriovenous fistula
- ICH
- intracranial hemorrhage
- MMA
- middle meningeal artery
- OA
- occipital artery
- SSS
- superior sagittal sinus
- STA
- superficial temporal artery
- WSS
- wall shear stress
SUMMARY
PREVIOUS LITERATURE:
Dural arteriovenous fistulas (DAVFs) are abnormal arteriovenous shunts located on or between the layers of the dura matter. Sinus thrombosis, infection, trauma, or surgery may be present preceding the formation of DAVFs. Venous hypertension has been hypothesized to be the key factor triggering the formation of DAVFs by increasing the expression of hypoxia-inducible factor-1 and vascular endothelial growth factor leading to angiogenesis. SSS-DAVFs and parasagittal DAVFs are often loosely classified under the umbrella term “SSS-DAVFs, convexity-DAVFs or falx-DAVFs.”
KEY FINDINGS:
Parasagittal DAVFs are nonsinus-type fistulas with shunting point(s) centered on the junctional zone of the bridging vein and the SSS leading to ipsilateral CVR, while SSS DAVFs are sinus-type fistulas with shunting point(s) centered on the sinus or parellel parasinus wall with bilateral CVR.
KNOWLEDGE ADVANCEMENT:
Transarterial embolization by using liquid embolic material via the MMA(s) can be used as the primary treatment strategy in most cases of parasagittal and SSS DAVFs. The use of balloon sinus protection is not necessary in most cases of parasagittal DAVF but likely to be required in SSS DAVFs.
Dural arteriovenous fistulas (DAVFs) are abnormal arteriovenous shunts located on or between the layers of the dura mater. DAVFs are uncommon, with the reported detection rates of up to 0.5 per 100,000 adults per year.1,2
Sinus thrombosis, infection, trauma, or surgery may be present preceding the formation of DAVFs. Thus, venous hypertension, with or without venous cerebral ischemia, has been hypothesized to be a key factor triggering the formation of DAVFs, by increasing the expression of hypoxia-inducible factor-1 and vascular endothelial growth factor leading to angiogenesis.3,4
DAVFs most commonly involve the transverse-sigmoid sinuses and cavernous sinuses, with superior sagittal sinus (SSS) involvement seen only in up to 5% of DAVF cases. SSS DAVFs and parasagittal DAVFs are often indistinguishably classified under the umbrella term of “SSS DAVFs, convexity-DAVFs or falx-DAVFs.” SSS DAVFs are sinus-type fistulas with shunt point(s) on the dura surrounding the sinus or parasinus wall, while parasagittal DAVFs are non-sinus-type fistulas with shunt point(s) on the dura mater in close proximity to the dural penetration of the bridging vein.5 In sinus-type DAVF, the sinus is often progressively compartmentalized and may eventually occlude, leading to cortical venous reflux (CVR) and congestion. In non-sinus-type DAVF, however, there is direct drainage to the cortical veins and thus invariably CVR.5
Both SSS DAVF and parasagittal DAVF are prone to aggressive clinical symptoms because they often have direct or indirect CVR. The reported presenting symptoms include weakness, numbness, headache, seizures, visual symptoms, less frequently dementia, gait disturbance, aphasia, and tinnitus. Based on a literature review of 31 cases of SSS DAVF in 20 publications, up to 42% of patients presented with intracranial hemorrhage (ICH).6
In this retrospective review of a single-center cohort study, we aimed to explore the clinical presentations, imaging characteristics and endovascular treatment strategies of these 2 (SSS versus parasagittal) DAVF subtypes. Specific imaging features differentiating these 2 distinct entities were analyzed to help with disease categorization. The clinical implications of differentiating these 2 DAVF subtypes including potential procedural complications and the need for sinus balloon protection during embolization are discussed in this study.
MATERIALS AND METHODS
We conducted a retrospective analysis of a prospectively collected vascular malformation database after institutional ethics board approval. We identified all cases of dural arteriovenous fistulas treated endovascularly between January 2017 and December 2022. The subgroups of patients diagnosed with SSS or parasagittal DAVF were selected and included in this review.
Age, sex, clinical presentation, angiographic findings, treatment strategies, angiographic outcome, clinical outcome and complications were reviewed and recorded in an anonymized manner. Pretreatment 6-vessel biplane conventional angiography with selective cannulation of bilateral ICAs, external carotid arteries, and vertebral arteries was performed in all patients before treatment for evaluation of the angioarchitecture. DAVFs were categorized according to the Borden classification (types I–III) and Cognard classification (type I–V). Endovascular treatment was considered the primary treatment option in all cases after initial angiographic assessment. Endovascular techniques, including arterial access (and venous access in some cases), embolization vessel selection and techniques, the use of balloon for dural sinus protection, immediate angiographic outcome, and periprocedural complications (up to 30 days postprocedure) were recorded. Imaging follow-up after treatment was obtained with either MRA, DSA (or both), or CTA.
Ethical Aspects
This study obtained ethics approval from the research ethics board of the University of Toronto with ID number 19–5018.4. Informed consent requirement was waived owing to the retrospective nature of the study.
RESULTS
Clinical and Imaging Characteristics
Of 155 patients with DAVF managed in our institution between 2017 and 2022, 19 patients (12.3%) were identified with a parasagittal or SSS DAVF. Among these 19 patients, 14 had a parasagittal DAVF, 4 had a SSS DAVF, 1 patient had both parasagittal and SSS DAVF, and 1 had parasagittal DAVF and torcular DAVF. The patient with both parasagittal and SSS DAVF had 2 distinct DAVFs centered at 2 separate segments, namely mid-parasagittal and posterior SSS segments, respectively, and was treated in 2 separate sessions. The patient with both parasagittal and torcular DAVFs was only treated for the parasagittal component. The Online Supplemental Data summarize the clinical and imaging characteristics of patients with parasagittal DAVF and SSS DAVF.
For the group of patients with parasagittal DAVF, the 2 most common clinical presentations were headaches (6/15, 40%) and pulsatile tinnitus (5/15, 33.33%). Only 1 (1/15, 6.67%) patient presented with ICH. Most (12/15, 80%) of the shunts were located along the middle third of the SSS, and in all cases (15/15, 100%) the fistulous points were located on the dura in proximity to the junctional zone between the bridging vein and SSS. Middle meningeal artery (MMA) feeders were present in all (15/15, 100%) cases, followed by occipital artery (OA) and superficial temporal artery (STA) feeders (10/15, 66.67%). Unilateral CVR, ipsilateral to the side of fistula, was present in 15/15 cases (100%). All fistulas were classified as Borden type III and Cognard type IV fistulas.
For the group of patients with SSS DAVF, visual disturbance was one of the main presenting symptoms (2/5, 40%). None of the patients presented with ICH. All 5 patients had preceding trauma to the vertex or underlying dural venous sinus thrombosis. All 5 patients had the shunting zone located at the middle third of the SSS, with the fistulous points on the sinus or parasinus wall. MMA, OA, and STA feeders were present in all 5 cases of SSS DAVF. All 5 cases of SSS DAVF were of Borden type II, 4 cases (80%) were of Cognard type IIa+b, and 1 case (20%) was of Cognard type IIa. Bilateral CVR was observed in all 5 cases.
Embolization Procedures
The Online Supplemental Data summarize the treatment and outcomes of patients with parasagittal DAVF and SSS DAVF.
Arterial access was obtained via transfemoral or transradial approaches. Venous access, if required, was done via transfemoral or transjugular approaches. Typically, a 5F to 8F guiding catheter was used, depending on vascular tortuosity and treatment approach.
Transarterial embolization was used as the endovascular treatment approach in all cases of parasagittal DAVF (15/15, 100%) and all except for 1 of the SSS DAVF cases (4/5, 80%).
Transarterial embolization with liquid embolic materials (typically Onyx, Covidien/ev3) was performed by using either flow-directed microcatheters (with or without detachable tip), dual lumen balloon microcatheters, or pressure-cooker techniques by navigating a second microcatheter more proximally to the same branch to build up a plug with coils and/or glue. The arterial access route for embolization was decided based on vessel tortuosity and the size of the vessels. Dural (rather than transosseous) arterial feeder with straighter course and larger diameter was first chosen for embolization due to the ease of catheter navigation. Injection of liquid embolic material was performed to occlude the fistulous point and the adjacent distal arterial feeders. Injection was stopped as soon as the liquid embolic material reached the foot of the vein or sinus wall, to minimize excessive reflux to the veins or sinus, especially if the veins or sinus are still used by the brain for normal venous drainage, to avoid venous thrombosis and propagation. Contrast opacification of the diseased vein or sinus only on the arterial phase but not the normal venous phase of the angiogram was indicative of its nonfunctioning status (Fig 1 and Fig 2).
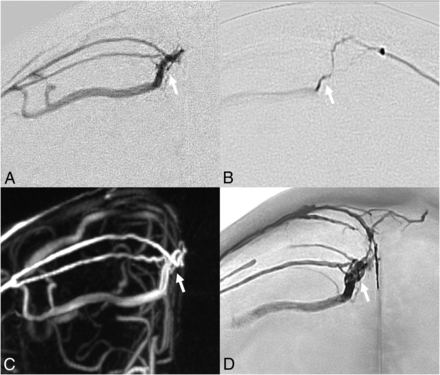
Eighty-three-year-old man with right parasagittal DAVF. A–C, Digital subtraction angiography, frontal view. D, Nonsubtracted fluoroscopic image. A–C, Arterial feeders from the right MMA and left MMA are centered on the junctional zone of the cortical bridging vein entering the SSS (fistulous point, arrow) with ipsilateral CVR. D, Transarterial Onyx embolization via the right MMA is evidenced by formation of Onyx cast along bilateral MMA arterial feeders and the diseased cortical vein.
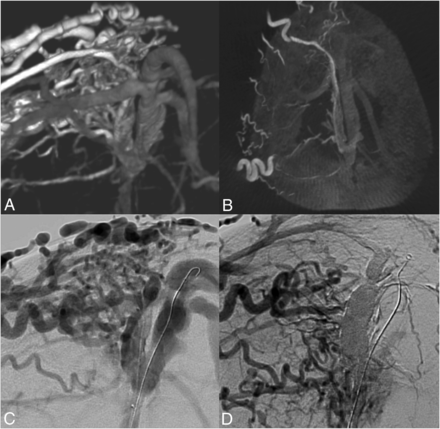
Thirty-four-year-old woman with SSS DAVF. A, B, and D, Digital subtraction angiography, frontal view. C, Nonsubtracted fluoroscopic image. A and B, Arterial feeders from the transosseous branches of bilateral OAs and STAs, left MMA, bilateral posterior meningeal arteries, arteries of Davidoff and Schechter, right anterior falcine artery, and left pericallosal pial-dural branch (not shown). The fistulous points are centered on the wall of the patent SSS, with extensive bilateral CVR (arrows) due to occluded right transverse sinus and hypoplastic left transverse sinus, precluding the use of balloon sinus protection. The SSS was used solely for fistulous drainage. C, 3 sessions of staged transarterial embolization, with Onyx cast formation along the sinus wall, the arterial feeders, and the draining cortical bridging veins. There is evidence of Onyx reflux into the SSS, nonocclusive. D, Postembolization angiogram confirms complete occlusion of the DAVF.
Balloon sinus protection during embolization was performed in 2 of 15 (13.33%) of parasagittal DAVF cases and 2 of 5 (40%) of SSS DAVF cases. Three of 5 (60%) cases of SSS DAVF in our series had embolization without balloon sinus protection; 1 had severely stenosed SSS caused by depressed skull fracture, 1 had chronically thrombosed SSS, and the other one had patent SSS but with challenging venous access (occluded right transverse sinus and hypoplastic left transverse sinus).
The only case treated by a transvenous approach was performed on a patient with SSS DAVF (Fig 3). The fistulous point was centered on a single channel of a bifurcated SSS. Liquid embolic embolization of the diseased sinus channel was performed under balloon protection of the dominant sinus channel.
Seventy-five-year-old man with SSS DAVF with variant morphology. A, C, and D, Digital subtraction angiography, frontal view. B, Axial CT reconstruction from spin angiography. A–C, Variant anatomy of SSS-split channel/unfused segment. The arterial feeders from bilateral MMAs, OAs, and STAs, centered on the wall of the right para-midline channel of SSS, with bilateral CVR. D, Single session of transvenous Onyx embolization under balloon protection of the left paramidline channel of SSS resulting in complete occlusion of DAVF.
Angiographic Outcome
Arterial feeders from the MMA (unilateral or bilateral) were present in all cases of parasagittal and SSS DAVF. For all cases of parasagittal DAVF, transarterial embolization with liquid embolic material via the MMA was attempted as the primary treatment approach. With this approach, complete angiographic occlusion was achieved in 93.33% (14/15) of parasagittal DAVF cases. A single case (1/15, 6.67%) of parasagittal DAVF required additional embolization via the STA feeder during the same session, rendering total complete angiographic occlusion after the first embolization session at 100% (15/15). Imaging follow-up was performed in 93.33% (14/15) of patients with parasagittal DAVF, 73.33% (11/15) of patients had DSA follow-up, and overall mean imaging follow-up duration was 11 months. Recurrence on follow-up angiogram was detected in 2 patients. One was re-treated successfully with repeat embolization that remained occluded with no recurrence at 2 years follow-up while the other patient who had both a parasagittal and SSS DAVFs had recurrence of the parasagittal DAVF and was not re-treated in view of minimal fistulous flow in an asymptomatic patient who is still recovering from scalp necrosis (result of liquid embolic reflux to the scalp vessels from embolization of SSS DAVF).
For cases of SSS DAVF, complete angiographic occlusion was achieved in 2 patients after the first treatment session (2/5, 40%). For complex SSS DAVF cases, staged embolization was anticipated by the operators with primary target of embolization predetermined before each session. One patient required a total of 3 staged embolization sessions to achieve complete angiographic occlusion while the other patient had complete occlusion after 2 staged embolization sessions with subsequent recurrence, requiring 3 additional embolization treatment sessions (a total of 5 treatment sessions). One patient had residual fistula fed by the anterior falcine artery, with resolution of CVR and clinical symptoms, not planned for further embolization. Imaging follow-up with DSA was performed in all 5 patients, with mean follow-up duration of 7.6 months. Four of 5 (80%) patients eventually achieved complete angiographic occlusion with no recurrence on final follow-up imaging.
Clinical Outcome and Procedural Complications
All patients in both parasagittal and SSS DAVF groups had improvement or resolution of clinical symptoms post embolization.
In the parasagittal DAVF group, 1 patient had a technical complication of a retained distal segment of a microcatheter (proximal to the detachable tip) in the external carotid artery following liquid embolic (Onyx) embolization via the MMA, with no clinical consequence. Another patient had inadvertent extravascular Onyx leakage surrounding the SSS during transarterial liquid embolic embolization via the MMA, which resulted in a thin (2 mm) subdural hematoma along the falx, with no clinical deficits and complete resolution on subsequent follow-up.
In the SSS DAVF group, 2 patients experienced scalp necrosis postembolization, attributed to penetration of liquid embolic materials to the scalp arterial feeders. One healed rapidly without infection while the other experienced a large area of occipital scalp necrosis complicated with infection and abscess formation requiring drainage and antibiotics. The latter patient had both parasagittal DAVF and SSS DAVF in 2 separate segments. He was treated initially for the parasagittal DAVF without complications. The SSS DAVF subsequently transformed into higher grade (Borden type II) fistula that prompted treatment. After pressure-cooker techniques via 2 occipital arterial feeders under balloon sinus protection, complete angiographic occlusion was achieved in a single session. However, the patient developed scalp necrosis postprocedure with superimposed infection required drainage.
DISCUSSION
In this retrospective study of 19 patients with 15 parasagittal DAVF and 5 SSS DAVF, our patient cohort has a mean age of 5th to 6th decades with male predominance, similar to the findings of the published meta-analysis on SSS DAVF.6 Different from the published meta-analysis that demonstrated a high rate (up to 42%) of ICHs,6 in our series we found a relatively low hemorrhagic rate (1/19, 5.26%). The exact reason for this discrepancy in hemorrhagic presentation is unclear and may be attributed to publication bias, small number of patients given its rarity, and increased detection rates in recent years with higher prevalence rates of imaging for nonspecific symptoms. Nevertheless, active treatment is recommended in all cases of parasagittal DAVF (Borden type III) and in most cases of SSS DAVF (Borden type II) given their inherent risk of subsequent neurologic deterioration and risk of hemorrhage.
In our study, we categorized our patients into 2 groups depending on the location of the shunt: parasagittal DAVF and SSS DAVF. Patients with parasagittal DAVF had their fistulous point(s) on the dura mater in close proximity to the dural penetration of the bridging vein (Fig 1). Patients with SSS DAVF, on the other hand, had their fistulous point(s) more medially on the dura surrounding the sinus wall (Fig 2) or parallel parasinus channel (Fig 3). In our study, we observed that in parasagittal DAVF, venous reflux was seen primarily along the cortical veins ipsilateral to the shunt point (as parasagittal bridging veins are limited by the dura propria that are adhered back-to-back along midline) and secondarily into the SSS in the presence of patent connection. In SSS DAVF, venous reflux was seen primarily along the SSS, with presence of cortical venous reflux bilaterally in higher grade fistula.
For parasagittal DAVFs, we found that the most common site of fistulous connection lies adjacent to the middle segment of the SSS. This occurrence may be explained by the following anatomic considerations. The bridging veins draining into the SSS are smaller in caliber along the anterior third of the SSS, and larger in caliber along the middle third of the SSS.7 Bridging veins join the middle segment of the SSS predominantly in a perpendicular or retrograde fashion (ie, <90° angle), while an antegrade course (or >90° angle) is more commonly observed in the anterior segment.8,9 Hemodynamic assessment by using computational fluid dynamics (CFD) based on bridging vein physical models demonstrated higher tendency for formation of thrombosis within the bridging vein when the diameter of a bridging vein is greater than 1.2 mm and the entry angle is less than 65° (retrograde course) due to significantly reduced wall shear stress (WSS).10 Low WSS causes sharp reduction of the anticoagulant substance, enhancement of leukocyte adhesion, and proliferation of smooth muscle that can lead to thrombosis. In addition, bridging veins in the middle segment of the SSS were more often found with hairpin loops or lacunae formation prior to its confluence with the superior sagittal sinus; these may cause flow turbulence or stagnation, increasing the propensity for thrombus formation.11 WSS in the bridging vein wall reduces more significantly (hence increases propensity for thrombus formation) compared with the SSS wall, which may explain the higher incidence of parasagittal DAVF (than SSS DAVF) in our cohort.
In all cases (15/15, 100%) of parasagittal DAVF, the fistulous points were located in proximity to the junctional zone between the cortical vein and the SSS, which, according to the CFD hemodynamic assessment described above, is the predilection site of cortical vein thrombosis.10 The predilection for occlusion of the junctional zone between cortical vein and the SSS is supported by the anatomy (change in caliber) and histology of the bridging vein at the junctional zone. There is focal dilation followed by narrowing (“puffy vein appearance”) before the confluence of cortical veins with the SSS.7 The collagen fibers of the distal cortical vein are densely packed along its subarachnoid course with longitudinally oriented fibers, but only loosely webbed along its subdural course leading to the junctional zone with the SSS, with circumferentially oriented fibers (constricted cuff segment) just proximal to its confluence with the SSS.9 These abrupt changes in caliber and histology may lead to flow disturbance, thrombus formation, and subsequent DAVF development. These features may be accentuated with arterialization because dilation of the cortical vein may promote further narrowing of this sphincter mechanism (Fig 1) leading to occlusion of the junction between the cortical vein and the SSS. Thus, we hypothesize that both a primary thrombotic occlusion of the junctional zone between the cortical vein and the SSS and the primary arterialization of a bridging vein may explain the imaging features of parasagittal dAVF with a fistula location at the junction between the cortical vein and the SSS as well as a missing or narrowed connection between these 2 venous structures.
Bridging vein thrombosis can be clinically silent due to abundant collateral circulation whereas SSS thrombosis causes backflow obstruction of all draining veins before the lesion location thus being more commonly clinically symptomatic.10 In our study, most (14/15, 93.3%) of the cases of parasagittal DAVF had no precipitating etiology, while all (5/5, 100%) patients with SSS DAVF had either prior trauma with skull fractures causing dural venous sinus stenosis or preceding dural venous sinus thrombosis. These conditions can lead to venous hypertension and can cause an increase in angiogenic growth factors which have been found to be related to the formation of DAVF.
In terms of treatment strategy, MMA arterial feeders were the primary route for transarterial embolization. With this treatment approach, complete angiographic occlusion was achieved in 93.3% (14/15) of parasagittal DAVFs. Balloon protection of the SSS during transarterial embolization of parasagittal DAVF was infrequently used and as highlighted by the anatomic considerations discussed above, in retrospect not deemed necessary. Reflux of liquid embolic material into the SSS during embolization was not observed in any of our cases as the connection between the cortical vein and the SSS was occluded or narrowed in all cases of parasagittal DAVF. We therefore propose that in parasagittal DAVFs, balloon sinus protection of the SSS during embolization, especially in cases of occluded or narrowed connection with the SSS, is not required. We observed no permanent complications for the treatment of parasagittal DAVF.
On the other hand, in SSS DAVF, more complex treatment strategies involving multiple treatment sessions or transvenous approaches, as well as complications (scalp necrosis) may be encountered. Based on our experience, the risk of developing scalp necrosis after embolization would depend on the amount of reflux of embolic material into the scalp arteries and the formation of collaterals to the affected scalp region. Tight band application over the scalp has been performed in a small number of patients in other centers to prevent excessive reflux of embolic materials, though its use has yet to be validated. As the shunting zone is located on the sinus wall with direct drainage to the SSS, we used balloon sinus protection in 2 of 5 (40%) of our SSS DAVF cases, thus preventing reflux of embolic material into the SSS. Balloon sinus protection was not used in the other 3 of 5 (60%) of our SSS DAVF cases due to occluded or stenosed SSS in 2 of 5 (40%) of the cases and due to challenging venous access with occluded right transverse sinus and hypoplastic left transverse sinus in 1 of 5 (20%) of the cases. The patient with challenging venous access had patent SSS, and without balloon sinus protection, there was reflux of Onyx into the SSS (nonocclusive in this case). We thus believe that balloon sinus protection is an important strategy in the treatment of SSS DAVF with patent sinus.
This study has limitations and inherent bias being a retrospective single-center study. Even though our series is from a high-volume neurovascular unit, the number of cases included in this study was limited. This shows that this is a rare pathology and therefore our results cannot be extrapolated to other lower-volume settings as such. A multicenter study with review of pooled data would be of value to validate our findings.
CONCLUSIONS
Our study found that the shunting point(s) of parasagittal DAVFs were centered on the junctional zone of the bridging vein and the SSS with ipsilateral CVR, while the shunting point(s) of SSS DAVFs were centered on the sinus or parallel parasinus wall with bilateral CVR. Transarterial embolization by using liquid embolic material via the MMA(s) can be used as the primary treatment strategy in most cases. In cases of parasagittal DAVF (commonly seen with occluded or stenosed connection of the involved bridging vein with the SSS), or in cases of SSS DAVF with occluded or stenosed sinus, our results suggest that the use of balloon sinus protection during embolization is not necessary. On the other hand, SSS DAVFs with patent sinus are likely to require balloon protection as these cases typically require complex treatment strategies or staged embolizations to achieve complete cure.
Footnotes
Dr. Krings acknowledges the generous support from the Patricia Holt-Hornsby and Dan Andreae Vascular Research Unit and UMIT (University Medical Imaging Toronto).
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received January 17, 2024.
- Accepted after revision March 11, 2024.
- © 2024 by American Journal of Neuroradiology