Abstract
BACKGROUND AND PURPOSE: Morning glory disc anomaly (MGDA) is a congenital malformation characterized by a funnel-shaped optic disc excavation with radiating vessels and a central glial tuft. Imaging is essential to evaluate associated cephalocele and steno-occlusive vasculopathy. The goal of this study was to assess optic nerve, chiasmatic, and sphenoid bone morphology in MGDA.
MATERIALS AND METHODS: This retrospective study examined all subjects with funduscopically confirmed MGDA diagnosed and imaged with brain MR imaging between 2008 and 2023.
RESULTS: Thirty-two children met inclusion criteria. Ocular involvement was unilateral in 29 subjects and bilateral in 3. Segmental optic nerve enlargement ipsilateral to the MGDA was seen in 21 subjects, with 3 also demonstrating a segmental reduction in the size of the ipsilateral optic nerve. Segmental reduction in the size of the ipsilateral optic nerve was present in 3 additional subjects, one with bilateral MGDA. The optic chiasm appeared asymmetrically thickened in 21 subjects, often with deformity. The optic nerves appeared normal in signal intensity in all subjects, with faint peripheral chiasmatic enhancement in 4 of 20 patients who received contrast. Optic nerve findings were stable in 15 subjects with multiple examinations. A persistent craniopharyngeal canal was identified in 17 subjects with sphenoid cephalocele in 1 and mild inferior pituitary gland displacement in 4. Tubular or nodular nasopharyngeal lesions were seen in 10 subjects. One subject had an off-midline sphenoid bone cleft, midbrain deformity, and abnormal thickening of and enhancement around the left oculomotor nerve; the oculomotor nerve finding was present in 1 additional patient.
CONCLUSIONS: MGDA often manifests with ipsilateral optic nerve thickening, leading to a potential misdiagnosis as optic glioma. MGDA is also commonly associated with a persistent craniopharyngeal canal with variable pituitary gland and infundibular deformity, cephalocele, and tubular or nodular nasopharyngeal lesions.
ABBREVIATIONS:
- MGDA
- morning glory disc anomaly
- MMD
- Moyamoya disease
- ON
- optic nerve
- PCPC
- persistent craniopharyngeal canal
Morning glory disc anomaly (MGDA) is a congenital ocular malformation that was first comprehensively described by Kindler,1 in 1970, who coined the descriptive term, with similar findings reported by Handmann,2 as early as 1929. MGDA is characterized by a funnel-shaped excavation of the optic disc with an overlying central glial tuft that resembles the central white of the morning glory flower.3,4 This rare entity affects approximately 2.6 of 100,000 children and is usually unilateral.5 Cases are mostly sporadic; however, associations with mutations in genes involved in ocular development (such as PAX6) have been described.6 The proposed pathophysiology is a primary mesoectodermal dysgenesis, in which there is partial development of the lamina cribrosa and incomplete closure of the embryonic fissure, leading to improper development of the posterior wall of the sclera.7,8
MGDA is diagnosed clinically and patients may present because of vision loss, new-onset strabismus, or leukocoria.9 It is important, though sometimes challenging, to differentiate MGDA from optic disc coloboma, which, unlike MGDA, has a robust genetic predisposition, requires different management, and carries a different prognosis.10,11 On MR imaging, MGDA is characterized by a funnel-shaped excavation of the optic disc, with adjacent hyperintense tissue on T1-weighted MR imaging, likely representing a chorioretinal pigmentary disturbance. There is typically effacement of the adjacent perioptic subarachnoid space, lack of the usual enhancement at the lamina cribrosa with discontinuity of the uveoscleral coat, and, less frequently, fatty infiltration of the optic nerve (ON) sheath, ON enhancement, and variable microphthalmia.3
MGDA is associated with a wide range of ocular, intracranial, and vascular abnormalities as well as midline skull base defects.5,12⇓⇓⇓–16 Recent studies reported the coexistence of a persistent craniopharyngeal canal (PCPC), with or without pituitary malformation and hypopituitarism.13,17 Doneda et al18 also reported 3 cases of MGDA with associated ipsilateral ON and chiasm thickening and dysplasia.
Previously, we described the characteristic imaging findings of MGDA on MR imaging.3 In this large, retrospective series that includes 6 subjects from the prior publication, we assessed MR imaging examinations to characterize the less well-described spectrum of ON, chiasmatic, and sphenoid bone anomalies. Furthermore, we sought to assess the morphology of a PCPC when present, the presence of pituitary gland deformity, and overt sphenoid cephalocele. Finally, we aimed to identify additional diagnostic features and shed light on the underlying pathogenic mechanisms involved in MGDA.
MATERIALS AND METHODS
Subjects
This was a retrospective study approved by the institutional review board (Boston Children's Hospital, Boston) (IRB-P00046220) and performed in accordance with the Declaration of Helsinki. The study population consisted of patients with funduscopically confirmed MGDA at the time of presentation, imaged from January 2008 to May 2023, who had MR imaging examinations of the brain and orbits.
Imaging Protocols
Patients underwent MR imaging by using either a 1.5T Signa TwinSpeed MR imaging scanner (GE Healthcare) or a 3T Tim Trio, Magnetom Vida, Magnetom Prisma, or Magnetom Skyra MR imaging scanner (Siemens). Examples of imaging protocols are shown in the Online Supplemental Data.
Imaging Review and Analysis
Images were independently reviewed using the hospital PACS by 1 staff pediatric neuroradiologist with 30 years of pediatric neuroradiology experience and a pediatric neuroradiology fellow. Findings were tabulated as follows: MGDA laterality, ipsilateral ON size, size and morphology of the optic chiasm, abnormality of the pituitary fossa/skull base such as a PCPC, basal encephalocele and pituitary gland and infundibular anomalies, and the presence of nasopharyngeal lesions. The ON and chiasm were assessed qualitatively; the contralateral side of the same patient was used as a reference for unilateral cases, and both sides were qualitatively assessed for bilateral cases. Evidence of Moyamoya disease (MMD) was also documented. We then calculated the numbers and percentages of these abnormalities.
Pathologic Examination
In a single case with surgical resection of tissue from the anomalous region in the nasopharynx, pathologic features were examined by a pediatric pathologist.
RESULTS
Study Population
Our study cohort consisted of 32 children, 17 males (53.1%) and 15 females (46.9%), ranging in age from 3 months to 16 years with a mean age of 5 years at the time of MR imaging. In our study, only 2 patients had confirmed genetic alterations; one had a single nucleotide variant in the beaded filament structural protein 2 (BFSP2) gene, and the other had a duplication in the 7p14.3 region 26. Twenty-nine (90.6%) children had unilateral MGDA (left/right, 19/10) and 3 patients had bilateral MGDA. The results are summarized in the Online Supplemental Data.
Imaging Findings
ON and Chiasm.
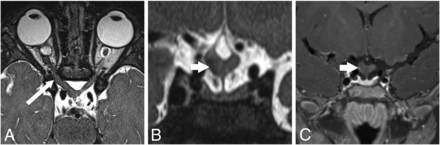
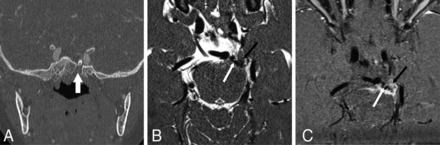
In 21 subjects (65.6%) with MGDA, the ipsilateral ON showed mild segmental thickening. This affected the intraorbital segment in 5 cases, the prechiasmatic segment in 5 cases, and both segments in 11 cases (Fig 1). The intraorbital ON appeared mildly small in 5 subjects (1 bilateral case with a contralateral large ON), and the prechiasmatic ON appeared mildly small in 1 subject. The ipsilateral prechiasmatic ON appeared large in 2 subjects. The optic chiasm appeared abnormal in 21 patients (65.6%), with thickening ipsilateral to the MGDA in 19 patients (59.4%) (Fig 1). In 12 patients, including 2 without chiasmatic thickening, the optic chiasm appeared tilted inferiorly on the side of the MGDA and/or deformed (Fig 1B). In 2 of 3 cases of bilateral MGDA, ON and chiasm thickening were more pronounced on the side of the more severe MGDA. Signal intensity on T2WI was normal for all ONs and chiasm in all subjects. Gadolinium-enhanced T1-weighted images (available for 20 subjects) showed faint peripheral enhancement around a thickened optic chiasm in 4 subjects (Fig 1C). Among 15 subjects with multiple examinations spanning 2 months to 11 years, the ON and chiasmatic findings remained stable.
ON and chiasmatic findings on MR imaging. A–C, P5; An 11-month-old girl. A, Axial T2 sampling perfection with application-optimized contrasts by using different flip angle evolution (SPACE sequence; Siemens) image shows a right MGDA with mild asymmetric right ON thickening (long arrow) without signal abnormality. B, Reformatted coronal T2 SPACE image reveals a chiasmatic deformity, asymmetrically thickened on the right (short arrow). C, Coronal contrast-enhanced fat-suppressed T1-weighted image shows minimal peripheral enhancement around the right side of the chiasm (short arrow).
Central Skull Base and Pituitary Gland.
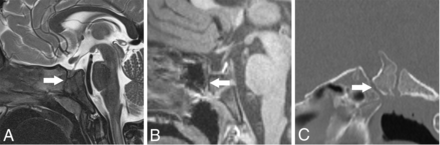
A PCPC was present in 16 cases (50%), with apposed margins in 9 (Fig 2) and 7 with ≥1-mm separated margins (Figs 2–4), with a small inferior pituitary fossa beak in 5. One additional patient had an off-midline cleft in the sphenoid body ipsilateral to the MGDA (Fig 5). Three subjects demonstrated a small beaklike protrusion inferior to the pituitary fossa without a visible PCPC. Of 15 subjects without a demonstrable PCPC, there was sphenoid sinus pneumatization extending inferior to the sella in 6 and low-resolution, thick slice 1.5T MR imaging in 1, precluding optimal assessment for a PCPC.
PCPC and pituitary fossa anomalies on MR imaging and CT. P3; A 14-month-old girl with bilateral MGDA. A, Sagittal T2-weighted MR imaging shows a PCPC with apposed margins (long, wide arrow) and minimal inferior pituitary fossa beaking. B and C, P25; A male patient with bilateral MGDA. B, Sagittal T1 MPRAGE MR imaging in a patient 5 years of age shows partial sphenoid pneumatization and a partially empty sella. The PCPC is difficult to appreciate (long, wide arrow) but is readily seen at 4 years of age on sagittal reformatted CT (long, wide arrow, C). Note the normal spheno-occipital synchondrosis dorsal to the PCPC.
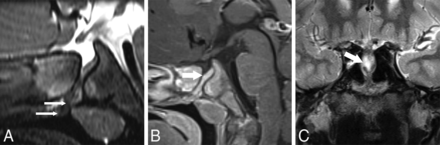
PCPC with a sphenoid cephalocele on MR imaging. A and B, P1; A 16-month-old boy with a right MGDA. A, Reformatted sagittal T2 SPACE sequence. B, Sagittal contrast-enhanced T1 SPACE images show a deep-set and angulated pituitary fossa associated with a wide PCPC containing pituitary tissue (wide white arrow). Note that in A, there is also tubular hypointense tissue that differs in signal intensity from pituitary tissue projecting into the nasopharynx inferior to the pituitary tissue (thin white arrow). C, P6; A 14-year-old boy with a right MGDA. Coronal T2-weighted image shows a sphenoid cephalocele containing CSF and pituitary gland (wide white arrow).
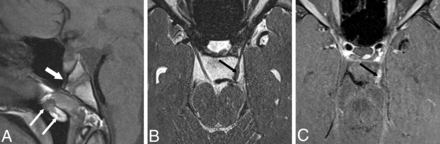
PCPC, tubular nasopharyngeal hamartoma, and oculomotor nerve abnormality on MR imaging. A–C, P7; A 15-year-old girl with a left MGDA. A, Sagittal T1-weighted image shows a deep-set pituitary fossa and PCPC (long, wide white arrow) and a heterogeneous part-fatty, tubular lesion protruding into the nasopharynx (long, thin white arrows). B, Axial T2 SPACE sequence MR imaging shows asymmetric thickening of the cisternal segment of the left oculomotor nerve (black arrow), ipsilateral to the MGDA. C, Axial contrast-enhanced fat-suppressed T1-weighted MR imaging shows avid oculomotor nerve enhancement (black arrow) that appeared stable at 2-year follow-up.
Off-midline left sphenoid bone cleft and oculomotor nerve abnormality on CT and MR imaging. A–C, P31; A 5-year-old girl with a left MGDA. A, 2D reformatted contrast-enhanced coronal CT image shows a sloping pituitary fossa with a left-sided sphenoid bone cleft (wide white arrow). B, Axial T2 SPACE sequence MR imaging shows a proximally thickened left oculomotor nerve that originates anomalously from the left side of the midbrain (black arrow) with a prominent tortuous vessel (thin white arrow) originating near the origin of the left superior cerebellar and posterior cerebral arteries (corroborated on MRA; not shown). C, Axial fat-suppressed contrast-enhanced T1-weighted MR imaging shows the tortuous vessel (thin white arrow) with surrounding abnormal enhancement encompassing the left oculomotor nerve root entry zone with enhancement of the proximal cisternal oculomotor nerve segment (black arrow). These findings appeared stable during a 6-year period.
One patient had a sphenoid cephalocele (Fig 3C). Four with PCPCs ranging between 1.5 and 3 mm in width had pituitary tissue extending into the upper aspect of the PCPC (Figs 2 and 3). Sloping of the pituitary fossa and/or deviation of the infundibulum was present in 7 subjects with a deep-set pituitary fossa in 5 (Fig 4).
Nasopharyngeal Lesions.
Twelve subjects had tubular or small nodular lesions projecting into the nasopharynx along the ventral margin of the adenoid, inferior to the sphenoid body (37.5%) (Figs 3A and 4A). In 2 of these patients, a definitive PCPC was not demonstrated on imaging. The largest lesion appeared tubular with heterogeneous signal intensity with fatty and enhancing components. Following surgical resection, histopathologic examination demonstrated a benign pedunculated polypoid lesion containing a small portion of pituitary tissue at its base. The remaining tissue was lined by ciliated columnar respiratory-type epithelium and contained adipose tissue, seromucinous glands, and swaths of smooth muscle associated with ganglion cells, resembling muscularis propria (Online Supplemental Data).
Other Findings
The subject with histologic analysis of the nasopharyngeal mass had asymmetric thickening and enhancement of the left oculomotor nerve ipsilateral to the MGDA that was stable for 2 years (Fig 4B, -C). Another subject with an off-midline sphenoid bone cleft had a deformity of the midbrain with abnormal thickening of and enhancement surrounding the left oculomotor nerve ipsilateral to the MGDA that was stable for 6 years (Fig 5B, -C). This patient also had a prominent tortuous vessel coursing through the enhancing material. One subject had hypogenesis of the corpus callosum. Steno-occlusive vasculopathy with Moyamoya collaterals was present in 6 subjects with 1 additional subject demonstrating mild unilateral ICA narrowing. A degree of vascular tortuosity was seen in 3 subjects.
DISCUSSION
In this retrospective study, we evaluated a cohort of patients with funduscopically identified MGDA and analyzed the less-well-described associated imaging features. The most common associated optic pathway finding was segmental abnormal thickening of the ON and thickening/angulation of the optic chiasm ipsilateral to the MGDA, without associated signal abnormality and with trace enhancement surrounding the optic chiasm in a minority of subjects.
Prior studies have reported an association between MGDA and ON and chiasmatic abnormalities. Ceynowa et al5 and Ellika et al3 each reported a case of ipsilateral ON enlargement, referred to as probable ON glioma but without specifying whether contrast enhancement was present. Bandopadhayay et al19 and Kinori et al20 also described 1 case each of ON enlargement with mild peripheral contrast enhancement, interpreted as an optic glioma. Similarly, Doneda et al18 reported 3 cases of clinically diagnosed unilateral MGDA with an ipsilateral ON and chiasmatic thickening on MR imaging, considered nonspecific. In a larger cohort, Poillon et al21 described 9 cases of optic pathway enlargement ipsilateral to the MGDA, with no change at a mean 3.1-year follow-up. The intraorbital ON and the optic chiasm were most frequently affected. The authors reported no contrast enhancement and homogeneous signal on T1-weighted and T2-weighted images.21 In our study, we found segmental ipsilateral ON and/or chiasmatic enlargement to be stable on long-term imaging follow-up and concluded that these findings are non-neoplastic in nature. This finding aligns with prior data showing no evolution of the ON abnormalities in patients with MGDA.21,22 Similarly, the uncommon occurrence of stable oculomotor nerve thickening and surrounding enhancement also presumably relates to a static developmental malformation rather than a neoplasm. To the best of our knowledge, these changes in the oculomotor nerve have not previously been reported in association with MGDA.
We believe ON and chiasm thickening is potentially underdiagnosed in MGDA. Previous studies have identified glial and fatty aggregates along the intraorbital ON within the distal sheath3,23,24 and also noted the presence of heterotopic muscle cells in abnormally developed eyeballs.25 These findings suggest that ON and chiasmatic thickening may be malformative, indicating abnormal development and likely a continuum of the spectrum of abnormalities associated with MGDA. While to date, no cases of MGDA associated with histologically-proved optic glioma have been reported, in rare cases in which ON thickening progresses or visual acuity deteriorates, the differential diagnosis of optic glioma or other non-neoplastic masses, such as hamartoma or choristoma, should be considered so that appropriate therapy can be provided.19,26,27
Five cases showed thinning of the ipsilateral ON at the site of the MGDA. Previous studies have reported ON and chiasm thinning contralateral to the MGDA, possibly suggesting anterograde degeneration due to ganglion cell deficit within the developing retina.5,24 However, the absence of atrophy in the ipsilateral ON and contralateral optic tract makes the assumption of anterograde degeneration less likely.18 Instead, the asymmetry should be interpreted as a thickening on the affected side.18 Nguyen et al22 described a single case of ON thinning ipsilateral to the MGDA. ON hypoplasia may present as an isolated anomaly or associated with midline cerebral structural defects, such as the absence of the septum pellucidum, agenesis of the corpus callosum, or pituitary gland abnormalities.28 Several genetic mutations have been linked to ON hypoplasia, including PAX6 gene mutations, which are associated with MGDA.6 In our study, only 2 cases showed confirmed genetic alterations: One had a single nucleotide variant in the BFSP2 gene, and the other had a duplication in the 7p14.3 region.29 Notably, these genetic alterations have not been previously described in patients with MGDA.
MGDA has been reported in association with a variety of midline malformations, including hypertelorism, cleft lip and palate, agenesis of the corpus callosum, basal encephalocele, persistent embryonal infundibular recess, and endocrinologic abnormalities involving the pituitary gland.17,30⇓⇓⇓⇓-35 In our cohort 64% of cases had a small beaklike projection off the pituitary fossa, which pointed to the PCPC. One-half of our subjects presented with a PCPC, most with apposed margins. The PCPC was most readily diagnosed on CT and on thin-section, high-resolution 3T MR imaging examinations performed before extensive sphenoid sinus pneumatization. The PCPC is a corticated tract along the midline of the sphenoid bone, extending from the sellar floor to the anterior-superior nasopharyngeal roof. The PCPC is believed to arise due to incomplete closure of the Rathke pouch, the precursor to the adenohypophysis.32,36⇓-38 It is essential to assess the PCPC for ectopic pituitary gland, encephalocele, and nasopharyngeal lesions, meriting endocrinology assessment and sometimes requiring surgical intervention.17,37
Pathologic evaluations of the nasopharyngeal masses associated with the PCPC have been described in a handful of publications, but histologic features have not been reported in detail. Masses have been variously described as “teratoma,” “epignathus,” and “dermoid.”37,39,40 Our histologic examination showed benign disorganized diverse tissue types, including ectopic ganglionated smooth muscle resembling the muscularis propria. Even though 2 of our 12 cases with nasopharyngeal lesions did not show clear PCPC on imaging, we suggest the term “PCPC-associated malformation” to help resolve the disparate terminology used previously and to convey the non-neoplastic, malformative nature of the mass.
To the best of our knowledge, this is the largest cohort of patients with MGDA with skull base midline abnormalities. On the basis of the overlapping critical periods of development for the frontonasal process, midfacial structures, and primordium of the eyes, MGDA is believed to emerge from a single insult occurring during forebrain induction. However, the nature of the embryologic defect remains the subject of debate.3,30 We found nasopharyngeal lesions in 10 subjects with PCPC, 5 with apposed margins and 1 patient with an off-midline sphenoid cleft. Searching for nasopharyngeal masses is important regardless of the PCPC morphology and potentially impacts the surgical approach.41⇓-43 There is also a continuum of abnormalities associated with the PCPC, ranging from mild protrusion of pituitary tissue into the craniopharyngeal canal to an overt sphenoid cephalocele.
While not the focus of this report, 7 subjects (21.9%) had MMD or mild steno-occlusive vasculopathy. MMD is a chronic, occlusive, and progressive cerebral vasculopathy that usually affects the supraclinoidal segment of the ICA and its main branches.44 Several reports have demonstrated the association of the MGDA and MMD, typically ipsilateral.31,32,45⇓⇓-48 It is believed that a deficiency during neuroectodermal genesis and subsequent mesodermal changes may be responsible for the association between these 2 entities.45 Other ophthalmic findings related to MMD include retinal vascular occlusion, visual field defects secondary to hemorrhage or ischemia, and optic disc abnormalities.45
This study demonstrates the inherent limitations of a single-center cross-sectional design, notably the absence of generalizability and the inability to establish causality. However, we contend that the rarity of our findings and associations mitigated these limitations. Moreover, our primary objective was to educate radiologists with supplementary findings that could help corroborate the diagnosis of MGDA.
CONCLUSIONS
Ipsilateral ON and chiasmatic thickening and deformity are commonly observed in MGDA and should not be mistaken for optic glioma. MGDA is associated with the PCPC, which becomes more challenging to diagnose as the sphenoid bone pneumatizes. Finally, a spectrum of PCPC-associated abnormalities exists, ranging from a thin tract with apposed margins to mild deformity and protrusion of pituitary gland into the PCPC, tubular or nodular PCPC associated nasopharyngeal malformations, and, less frequently, a sphenoid cephalocele.
Footnotes
Drs. Firouzabadi and Soldatelli are co-first authors.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received January 9, 2024.
- Accepted after revision March 18, 2024.
- © 2024 by American Journal of Neuroradiology