Abstract
BACKGROUND AND PURPOSE: Anomalies of the corpus callosum are rare. Routine scanning in midtrimester of the pregnancy often fails to identify defective development. The purpose of the study was to identify the pericallosal artery and all its main branching arteries during early gestation from the first trimester onward, to measure the length of the pericallosal artery during its development, and to establish a normal vascular map for each week of development.
MATERIALS AND METHODS: We performed a single-center prospective, longitudinal clinical study in 15 patients between 11 and 22 weeks of gestation. The origin and course of the different blood vessels were identified.
RESULTS: There was a linear association among gestational age, the biparietal diameter, and the length of the pericallosal artery. The curvature of the developing pericallosal artery increases linearly with the gestational age and biparietal diameter, and 4 variations of branching of the callosomarginal artery were observed.
CONCLUSIONS: The pericallosal artery and its branches can be identified and measured from 11 weeks on, and the pericallosal artery takes its characteristic course. A defective course or an abnormal biometry of the pericallosal artery could be an early sonographic marker of abnormal development of the corpus callosum.
ABBREVIATIONS:
- BPD
- biparietal diameter
- CC
- corpus callosum
- CMA
- marginal callosal artery
- L1 and L2
- the anterior and distal part of the pericallosal artery to the highest point
The development of the corpus callosum (CC) starts with the formation of the genu during the eleventh week of gestation and progresses in an anterior-to-posterior direction with the development of the body and splenium.1 Finally, the most anterior part, the rostrum, is formed.2,3 More recent neuroimaging studies have shown callosal connections originating more centrally in the hippocampal primordium near and superior to the anterior commissure.4 The expansion of the lobes makes the anterior border of the CC move progressively forward to coincide with the enhanced anterior curvature of the cingulate gyrus.3 Finally, the rostrum and the genu connect the frontal lobes, the body of the CC joins the posterior part of the frontal lobes and the parietal lobes, and the splenium unites the temporal and occipital lobes.
Traditionally in the second trimester of pregnancy, the fetal brain is examined in 3 axial planes.5,6 Absence of the cavum septi pellucidi, an interruption of the cerebral falx, and absence of a transverse hypoechoic communication between the 2 frontal hemispheres are indirect sonographic signs of absence of the CC.
Because anomalies of the CC are rare (0.3%–0.7% to 2%–3%), their detection in a nonselected population remains difficult.7,8 Furthermore, routine axial scanning planes fail to identify defective development of the CC before midgestation.5,6,9 However, direct and complete visualization of the CC and pericallosal arteries can be established in the sagittal plane from 18 weeks on,10 though the fetal position, maternal obesity, and oligohydramnios may limit an optimal view in a sagittal plane.
High-resolution transvaginal sonography probes allow examining the central nervous system and diagnosing pathologic conditions at aneuploidy screening at 11–14 weeks.11⇓⇓⇓–15 Nevertheless, in a retrospective analysis of >45,000 pregnancies scanned between the eleventh and thirteenth week of gestation, none of the 10 cases of agenesis of the corpus callosum were either suspected or diagnosed.16
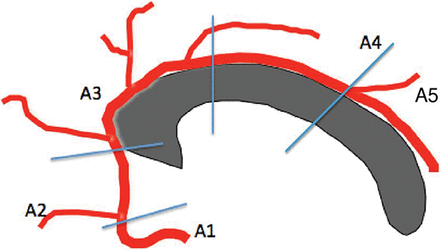
The CC is lined by the pericallosal arteries, which branch distally from the anterior cerebral artery. These vessels are divided into 5 segments as presented in Fig 1.
A1 is the segment originating from the internal carotid artery and extending to the anterior communicating artery. A2 extends from the anterior communicating artery to a region between the rostrum and genu. The A3 segment courses around the genu to the rostral part of the body. A4 and A5 segments are the continuation of the pericallosal artery.
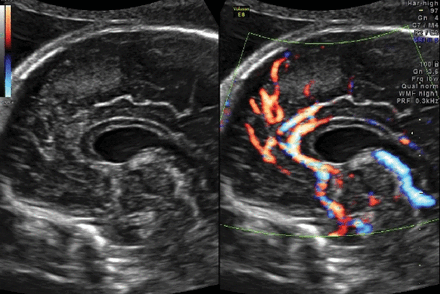
Power Doppler flow demonstrates the normal distribution of the pericallosal artery and its variant branching at the twentieth-week sonographic examination and more recently even at the end of the first trimester.17
The sensitivity of screening for fetal CC agenesis using indirect sonographic signs such as an abnormally shaped or absent cavum septi pellucidi (or ventriculomegaly/colpocephaly from midgestation onward) is poor5,6 and is not applicable in the first trimester. Furthermore, dysgenesis of the CC could escape detection because most of the aforementioned signs are lacking.17
Aim
In the absence of specific screening tools for and direct visualization of the developing CC before midgestation, we hypothesized that the progressive development of the pericallosal vascularization precedes callosal development and might therefore act as a marker for the early callosal development.
The blood supply of the corpus callosum is ensured by 2 arterial systems. The carotid system supplies the pericallosal artery. A part of the splenium is supplied by the vertebrobasilar system by its terminal branches. These systems give rise to perforating arteries that ensure the intrinsic vascularization of the corpus callosum, creating a system of regular stitches around the fibers of the corpus callosum.41,42,43 The formation of the corpus callosum is associated with medial and upward rotation of the cingulate gyrus, with consequent formation of the cingulate sulcus. When the CC does not form, the cingulate gyri do not rotate and are small due to hypoplasia of the cingulum, and the medial hemisphere sulci radiate to the third ventricle. An abnormal pattern might be an early indirect sonographic marker.
Therefore, dysgenesis of the corpus callosum could be reflected by a misshapen or abnormal course of the pericallosal arteries and their branches.18⇓–20 With this study, we aimed to document the normal longitudinal development and variants of the pericallosal vasculature from 11 to 22 weeks of gestation using power Doppler flow and high-frequency sonography probes.
Materials and Methods
We performed a single-center prospective, clinical study including 15 patients referred for sonographic examination at 11–13 weeks of gestation. Patients were eligible in case of a viable singleton pregnancy with a low first-trimester aneuploidy risk (<1/1000), no subsequent chromosomal abnormalities or growth restriction, and no sonographic evidence of fetal anomalies. Gestational age was determined by an early dating scan.21
Patients younger than 18 years of age or with multiple pregnancies were excluded. Eligible patients were invited for a weekly or biweekly follow-up scan by a single Fetal Medicine Foundation–certified operator up to 22 weeks of gestation, the time at which the fully developed corpus callosum could be identified by sagittal scanning of the fetal brain.
All patients underwent a second-trimester sonography by another sonographer who demonstrated a normal corpus callosum in a midsagittal view.
Transabdominal sonography was performed with a Voluson E8 Expert, (GE Healthcare, Milwaukee, Wisconsin) with a transabdominal RAB 4–8 D transducer (GE Healthcare).
A complete fetal biometry and a first-trimester aneuploidy screening were performed. The midsagittal plane used for identifying the nasal bone and nuchal translucency served as a template for the high-definition power color Doppler investigation of the pericallosal region in accordance with the as low as reasonably achievable principles. Settings were the following: armonics-high, speckle reduction imaging II 3, frequency mid, wall motion filter low, pulse rate frequency 0.6 kHz, persistence high).
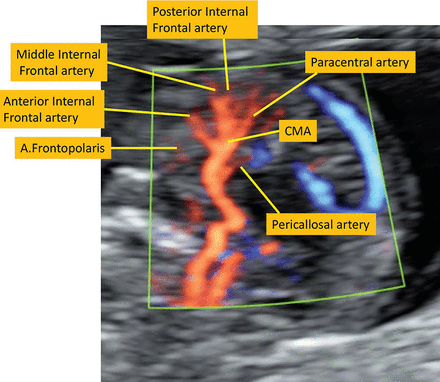
The thermal and mechanical indices were kept below 1 for safety reasons according to the recommendations of the Bio-Effects and Safety Committee of the International Society of Ultrasound in Obstetrics and Gynecology.22 The origin and the course of the frontopolar artery, the ramus anterior, the ramus medianus, the ramus posterior, the callosomarginal artery, the paracentral artery, and the precunealis were identified (Fig 2). Corresponding images and clips were digitally stored.
The callosomarginal artery is the largest branch of the pericallosal artery. The main branches are the frontopolar artery (A.Frontopolaris), the anterior internal frontal artery, the middle internal frontal artery, the posterior internal frontal artery, and the paracentral artery. They may arise from the pericallosal artery or the CMA.
To define the natural course of the pericallosal arteries in relation to the fetal head, we measured the distance between the frontonasal junction and the origin of the pericallosal artery in a sagittal plane. The length of the pericallosal artery was measured by drawing a straight line connecting the most anterior to most posterior part of this artery as visualized by color Doppler flow at 94 different time points. The mean and the fifth and ninety-fifth percentiles were calculated for the length of the pericallosal artery in relation to gestational age and biparietal diameter (BPD).
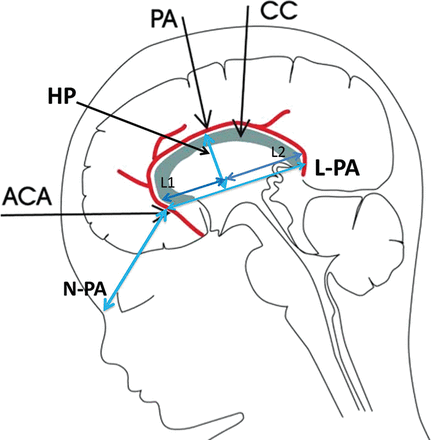
We defined the rounded course of the pericallosal artery by measuring the distance between the highest point of the curvature of the pericallosal artery perpendicular to the straight line connecting the most anterior to most posterior part of this artery as visualized by color Doppler flow. Subsequently, the length of the pericallosal artery distance was divided into L1 and L2, the anterior and distal part of the pericallosal artery to the highest point (Fig 3). Each measurement was performed 3 times and averaged. Mean values for the highest point, L1 and L2, with the fifth and ninety-fifth percentiles, were calculated for each gestational week.
N-PA indicates the distance between the frontonasal junction and the origin of the pericallosal artery; L-PA, the length of the pericallosal artery: a straight line connecting the most anterior and posterior part of this artery; HP, the highest point of the curvature of the pericallosal artery perpendicular to L-PA line; L1, the anterior part of the L-PA distance to the HP; L2, the posterior part of the L-PA distance to the HP; ACA, anterior cerebral artery.
To study the natural variation in the course and the origin of the marginal callosal artery (CMA), we constructed a diagram of the vascular development. The distances between the origins of the different branches of the pericallosal artery were measured for each gestational week. The assessment of the variants in the origin of the CMA was performed according to the Fisher classification.23
Normal fetal development was confirmed by systematic evaluation of the fetal anatomy and biometry (biparietal diameter, occipitofrontal diameter, head circumference, abdominal circumference, and femur length) and the presence of a normally developed corpus callosum and pericallosal artery at the second-trimester sonography by another independent Fetal Medicine Foundation operator. The pericallosal artery and its branches were reassessed on the anonymized stored images at 3 and 6 months to calculate the interobserver variability.
Statistical analysis was performed with SAS 9.3 (SAS Institute, Cary, North Carolina). Descriptive statistics are presented as mean (±SD) or median (percentiles), depending on the normality of the data. Frequency tables with the Fisher exact test were used to compare independent proportions. Linear regression between variables was performed, and the Pearson correlation coefficient was reported, together with its 95% confidence interval. Statistical significance was considered at the .05 significance level. Bland-Altman plots were used to evaluate the intraobserver variability. The study was approved by the ethics board of the University Hospital Leuven, Belgium, and all participating patients consented.
Results
All 15 consenting and participating patients completed the study. A total of 94 transabdominal observations were performed, with a median of 7 sonography scans per patient (range, 3–8). One patient was seen on 3 occasions in early pregnancy. Further management of her pregnancy occurred in another center. However, sonographic evaluation in the third trimester did not reveal CNS abnormalities in any of these patients (Table and Fig 4).
Number of observations in relation to gestational age. Mean length of the pericallosal artery (millimeter) in relation to gestational age (±SD)
The different branches of the pericallosal arteries at week 12, week 14, week 16 and week 18, respectively.
All patients delivered at term (mean, 39 weeks 1/7 days). The mean maternal age was 31.2 ± 4.4 years, and the mean body mass index in this selected group of 15 patients was 24.3 ± 3.2. The mean BMI allowed a successful visualization of the blood vessels. In 8/15 patients, the placenta was localized anteriorly. The pericallosal vasculature was assessed in short scanning episodes for an additional total scanning time of <60 seconds in all cases. The anterior part of the pericallosal artery could be observed in a sagittal plane from a crown-rump length of >36 mm (corresponding to 10 5/7 weeks of gestation).
In 4 of 15 patients with a sonogram at 11.0–11 6/7 weeks of gestation, the presence of a pericallosal artery could be demonstrated. At 12.0–12 6/7 weeks of pregnancy, the anterior part of the pericallosal artery was visualized in all but 1 patient (14/15). The anterior internal frontal artery, the middle internal frontal artery, and the posterior internal frontal artery were detected in 93.33% (14/15), 66.66% (10/15), and 26.66% (4/15) of patients, respectively.
From 13 to 13 6/7 weeks onward, the anterior internal frontal artery was seen in all cases (14/14). The middle internal frontal artery and posterior internal frontal artery were seen in 85.7% (12/14) and 75% (9/12), respectively. At 14–14 6/7 weeks, the middle internal frontal artery was depicted in all cases and the posterior internal frontal artery was seen in 78.57% (11/14). All the branches were demonstrated from 15 completed weeks onward in all patients. The precuneal artery (Fig 5) was observed from 16 weeks onward in 46.6% (n = 7) and by 20 weeks in 73.3% (n = 11). In 20% of the cases (n = 3), the middle and posterior internal frontal arteries were connected by a common trunk, the most common variant.
The precuneal artery (arrow) can be observed from 16 weeks onward, and this artery was visible in midtrimester in 80% of our fetuses.
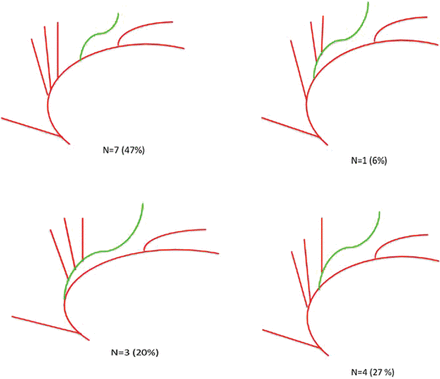
Variations of branching of the callosomarginal artery come in 4 different types, of which the CMA starting from the A1 and the A4 segment was not observed. From the CMA, 6 (40%) started in the A2 segment and 8 (53.3%) originated in the A3 segment. In 3 cases (20%), the CMA branched before the ramus anterior; in 1 case (6%), between the ramus anterior and ramus medianus; in 4 cases (27%), between the ramus medianus and posterior; and in 7 cases (47%), after the ramus posterior (Fig 6).
Variants of the callosomarginal artery.
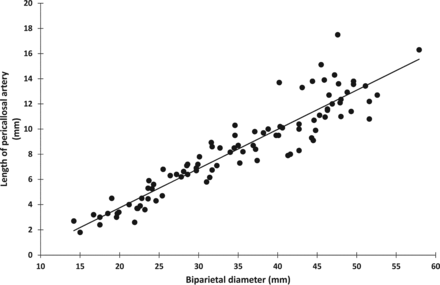
We found a strong linear correlation (P < .0001 in all cases) between the length of the developing pericallosal artery and gestational age (n = 94) (r = 0.951) (Fig 7), BPD (n = 94) (r = 0.932) (Fig 8), head circumference (n = 94) (r = 0.937), femur length (n = 94) (r = 0.933), and crown-rump length (n = 23) (r = 0.796).
Linear correlation between the developing pericallosal artery and gestational age (n = 94) (r = 0.951) (P < .0001).
Linear correlation between the developing pericallosal artery and BPD (n = 94) (r = 0.932) (P < .0001).
The distance from the nasofrontal junction to the origin of the pericallosal artery and the curvature of the developing vasculature documents its spatial development and that of the future CC. Between 12 and 22 weeks of gestation, the origin of the pericallosal artery distance increased linearly with gestational age (r = 0.905) and the BPD (r = 0.867).
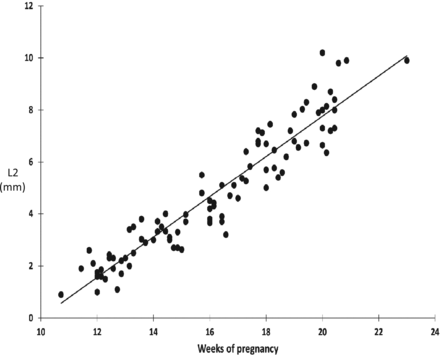
The curvature of the developing pericallosal vasculature was defined by the highest point (Fig 9), L1 (Fig 10), and L2 (Fig 11), all of which increased linearly with gestational age (r = 0.904; 0.935, and 0.944, respectively) and the BPD (r = 0.873; 0.926; 0.913). The highest point/L1 ratio, representing the slope of the anterior part of the pericallosal artery and hence the developing CC, decreased slightly throughout the investigated timeframe.
Height of the slope (millimeters) in relation to gestational age (n = 94) (r = 0.904) (P < .0001). HP indicates highest point.
Increasing slope of the pericallosal artery (millimeters) in relation to gestational age. (n = 94) (r = 0.935) (P < .0001).
Decreasing slope of the pericallosal artery (millimeters) in relation to gestational age (n = 94) (r = 0.944) (P < .0001).
We measured the distances between the different developing branches across time. The frontopolar artery and the ramus anterior, the ramus anterior and the ramus medianus, the ramus medianus and the ramus posterior, the ramus posterior and the callosomarginal artery, the callosomarginal artery and the paracentral artery, and the paracentral artery and precuneal artery illustrate the proportional growth of the pericallosal blood vessels. Intraobserver variations of the length of the pericallosal artery and the distances between the origins of the different branches were not statistically significant.
Discussion
Agenesis and dysgenesis of the corpus callosum are the more frequent central nervous system malformations associated with variable prognosis. In association with chromosomal abnormalities, genetic syndromes, and central nervous system and non-CNS abnormalities, the prognosis is invariably poor.24 Isolated complete agenesis, however, seems to have a better prognosis than a partial or hypoplastic corpus callosum.19 A recent meta-analysis of 27 studies on the outcome shows a higher proportion of chromosomal anomalies, more gross and fine motor control affection, and a higher percentage of epilepsy in the partial agenesis group compared with the complete agenesis of the CC group.40 Today, diagnosis relies on a midtrimester sonographic examination potentially revealing ≤1of the associated signs such as mild ventriculomegaly, colpocephaly, absent cavum septi pellucidi, upward displacement of the third ventricle, tear-drop configuration of the lateral ventricles, or cystic dilation of the third ventricle.25,26 However, these signs might be subtle or missing.27,28 The presence of a normal CC and its biometry has been assessed by transabdominal and transvaginal 2D and 3D sonography and with fetal MR imaging from 18 weeks onward.10,17,29⇓⇓⇓–33
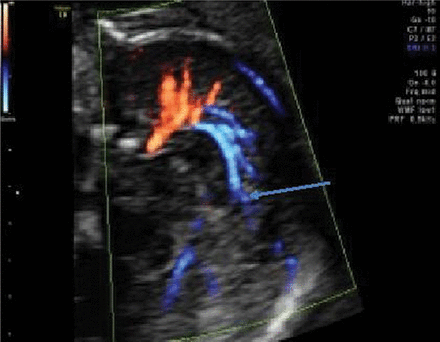
Indirect appreciation of the developing CC will be seen by demonstration of the pericallosal artery and its branches. Recently, color Doppler mapping documented the modified arterial vascular supply with loss of the semicircular loop in CC agenesis (Fig 12).25 In a partial agenesis of the CC, the paracentral artery follows the anterior part of the CC but loses its normal course when the CC vanishes. At this level, the artery moves in an upward and posteriorly oblique direction (Fig 13).19,20 The corpus callosum formation is associated with a medial and upward rotation of the cingulate gyrus, with consequent formation of the cingulate sulcus. In cases of an absent CC, the cingulate gyri do not rotate and are small due to hypoplasia. In cases of partial agenesis, we expect therefore a shorter length of the pericallosal artery as well as a different branching pattern and course. Knowledge of the development and variations in the different branches may enhance the diagnosis of partial agenesis of the corpus callosum.
Pericallosal artery and branches in agenesis of the corpus callosum.
Aberrant pattern of the pericallosal artery and its branches in partial agenesis of the corpus callosum.
In the first trimester, the midsagittal plane of the fetal head allows investigating the nasal bone and the nuchal translucency as screening markers for Down syndrome and the intracranial translucency for the detection of open neural tube defects.34,35 Adding power color Doppler flow in fetuses at rest for short time intervals, respecting thermal index and mechanic index, shows the developing pericallosal vasculature and its variants36⇓–38 in the first trimester either with 2D or 3D sonography.
Most first-trimester studies document either the presence or the course and/or length of the pericallosal artery only. In a cross-sectional study including 80 patients attending for first-trimester aneuploidy screening, chorionic villus sampling, or amniocentesis, a reference range of the length of the pericallosal artery was provided from 14 weeks onward in relation to BPD and gestational age.36 In agreement with Pati et al,36 we also detected a high linear correlation (>0.9) between the length of the pericallosal artery and gestational age and the BPD, respectively. However, in that study, the developing vascular map was not analyzed. Conturso et al38 viewed the pericallosal arteries in healthy fetuses at 11–13 weeks of gestation in 70 cases using 3D technology in the first trimester of pregnancy.
Diaz-Guerrero et al37 evaluated 150 fetuses between 11 and 14 weeks and failed to visualize the pericallosal artery in only 6 cases. Subsequently, 2 of these 6 cases were diagnosed with agenesis of the corpus callosum in association with a chromosomal abnormality. In the 4 other fetuses, the pericallosal artery was not seen due to the fetal position and excessive fetal movement.37 However, in addition to the biometry of the pericallosal artery, we favor evaluating the morphology of the vasculature of the pericallosal artery and its branches because it might enhance the diagnosis of complete agenesis as well as dysgenesis of the CC. This evaluation has already been described in the second trimester of pregnancy.20 Therefore, detailed knowledge of the arterial supply of the corpus callosum might distinguish normal variants from deteriorated vascularization associated with abnormal development of the CC.17,39
Limitations of our study are the small number of healthy subjects, therefore the lack of an unhealthy case, and an average body mass index of 24, which does not always represent the general population.
Conclusions
In a population of healthy fetuses, the pericallosal artery and its branches can be consistently identified and measured from 11 weeks on. A defective course or an abnormal biometry of the pericallosal artery could be an early sonographic marker for identifying abnormal development of the corpus callosum. Further prospective evaluation of the vascularization and biometry of the pericallosal artery in the late first trimester is needed for proof of this concept.
Acknowledgments
We sincerely thank the patients for the participation in this study.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received August 15, 2017.
- Accepted after revision October 30, 2017.
- © 2018 by American Journal of Neuroradiology