Graphical Abstract
Abstract
BACKGROUND: Brain AVMs are congenital anomalies of the cerebrovascular system, often discovered incidentally or through symptomatic presentations such as intracranial hemorrhage, seizure, headache, or neurologic deficits. Various treatment modalities exist for AVMs, including radiosurgery, a treatment technique that is noninvasive and efficient. Accurate imaging is crucial for risk assessment, treatment planning, and monitoring of these patients before and after radiosurgery.
PURPOSE: Currently, DSA is the preferred imaging technique. Despite its efficacy, DSA is notably invasive, presenting inherent risks to the patients. This systematic review and meta-analysis aimed to evaluate the efficacy of MRI sequences for monitoring brain AVMs after radiosurgery.
DATA SOURCE: We performed a comprehensive search of PubMed, Scopus, Web of Science, and EMBASE databases and a methodologic quality assessment with the QUADAS-2 checklist diagnostic test accuracy.
STUDY SELECTION: According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, 3,220 abstracts were screened, 98 articles were reviewed in full text, and 14 articles met the inclusion criteria.
DATA ANALYSIS: We used the bivariate random-effects meta-analysis model with STATA/MP 17 software for data analysis.
DATA SYNTHESIS: No publication bias was detected. Fourteen studies were eligible for qualitative and quantitative analysis. MRI offers high sensitivity (85%) and specificity (99%) in detecting residual AVMs. Different MRI sequences, including 3D TOF-MRA, 4D MRA, and arterial spin-labeling (ASL) demonstrated varying diagnostic accuracies with areas under the curve of 0.92, 0.97, and 0.96, respectively. 4D MRA had a sensitivity of 72% and specificity of 99%, ASL showed a sensitivity of 90% and specificity of 92%, while 3D TOF-MRA had 90% sensitivity and 87% specificity.
LIMITATIONS: Meta-regression did not fully explain the sources of heterogeneity. Only 1 study assessed the susceptibility-weighted angiography (SWAN) method, and most studies involved small participant groups with varied MR techniques and sequences. Additionally, the retrospective nature of most studies may introduce bias, warranting cautious interpretation of the results.
CONCLUSIONS: MRI sequences show acceptable diagnostic performance in postradiosurgery monitoring of brain AVMs, with ASL and 4D MRA showing acceptable diagnostic accuracy. Combining different MRI sequences may further enhance diagnostic reliability. However, further investigation is needed to assess whether MRI sequences can serve as a feasible substitute for DSA, considering their risk-benefit profile, with the potential to establish them as the recommended standard.
ABBREVIATIONS:
- AUC
- area under the curve
- ASL
- arterial spin-labeling
- DOR
- diagnostic OR
- SROC
- summary receiver operating characteristic
- SWAN
- susceptibility-weighted angiography
Brain AVMs are congenital anomalies of the cerebrovascular system, consisting of feeding arteries, nidus, and draining veins.1 AVMs are typically asymptomatic or found incidentally during neurologic symptom evaluations.2 Hemorrhage is a common presentation with an incidence rate of 1%–33%, followed by seizure (10%–30%),3,4 chronic headaches (6%–14%), and neurologic deficits due to hemodynamic changes or mass effect (3%–10%).5 Radiosurgery, microsurgical resection, endovascular embolization, or a combination of these methods is the treatment choice for brain AVMs. Surgical resection and endovascular embolization lead to a more rapid cure of AVMs by instantly removing or obliterating the AVM nidus, respectively. In comparison, stereotactic radiosurgery gradually obliterates the nidus during 2–3 years, necessitating careful follow-up imaging due to the latency period between the administration of stereotactic radiosurgery and the complete obliteration of the AVM.6
DSA is the criterion standard for interval monitoring of the AVM size, location, and blood flow patterns across time. Nonetheless, its diagnostic superiority is tempered by the invasive nature of the procedure, which carries inherent risks of neurologic complications.7,8 Conversely, MRI has emerged as the favored initial technique for screening purposes. MRI provides excellent soft-tissue contrast resolution, avoids ionizing radiation, and by using non-contrast sequences, MRI eliminates the risk of complications related to contrast agents. DSA is routinely accepted for monitoring treated AVMs, but recently different MRI sequences have been used for follow-up as a noninvasive technique to substitute for the follow-up DSA examination.7 TOF-MRA is the standard noncontrast method, albeit lacking comprehensive hemodynamic information. Arterial spin-labeling (ASL), as a perfusion MRI technique9 and 4D MRA as a dynamic contrast-enhanced method with subsecond temporal resolution have gained attention, because they provide dynamic flow information.10,11 Given the heterogeneous findings in the literature concerning AVM imaging techniques and their effectiveness, the objective of this systematic review and meta-analysis was to consolidate findings from relevant studies to assess and contrast the efficacy of diverse imaging modalities.
MATERIALS AND METHODS
Our study has been registered in the International Prospective Register of Systematic Reviews, under the registration number CRD42023475973. This document follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses of Diagnostic Test Accuracy Studies (PRISMA-DTA) guidelines, as cited in the references.12,13
Search Strategy
A comprehensive systematic search was performed across 4 leading electronic medical literature databases: PubMed, Scopus, Web of Science, and EMBASE. The search criteria were built on keywords related to “arteriovenous malformation,” “radiosurgery,” and “MR imaging.” Our comprehensive review encompasses scholarly publications up to March 2024. Detailed search strategies for each database are described in the Online Supplemental Data.
All findings are exported to Endnote Desktop software (https://endnote.com/downloads/) to organize and eliminate duplicate entries. Additionally, Review Manager (RevMan, Version 5; https://revman.cochrane.org/info), developed by The Cochrane Collaboration in 2020, was used to manage data related to the systematic review. Furthermore, 2 independent researchers manually reviewed the reference lists of the obtained full-text articles to ensure that we did not overlook any additional studies. Inclusion and exclusion criteria are described in the Online Supplemental Data.
Study Selection
Two independent researchers, M.A. and M.S., screened the abstracts and titles of studies to identify those meeting the inclusion criteria, working blind to each other’s selections. Discrepancies were resolved through discussion with M.T., ensuring that consensus was reached on the final selections.
Data Extraction
Researchers D.Z. and M.T. reviewed the full texts of selected articles, extracting qualitative and quantitative data. This review included general study details (publication year, country, study design), participant characteristics (demographics, follow-up period, MRI sequence type such as ASL MRI, 4D MRA, TOF-MRA, susceptibility-weighted angiography [SWAN], clinical presentation, AVM location, reference standard, interval between MRI and the reference standard), and outcome metrics (numeric data on patients with residual AVMs or complete obliteration, information for creating a 2 × 2 contingency table).
Studies provided sufficient quantitative data on diagnostic test performance, including the true-positive, false-positive, true-negative, and false-negative values, qualifying them for the quantitative synthesis phase (meta-analysis). In studies that evaluated multiple sequences, data for each sequence were independently included in the relevant meta-analyses based on 4 subgroups: 1) 3D TOF, 2) 4D MRA (contrast enhanced-dynamic), 3) ASL, and 4) SWAN. When multiple radiologists provided independent diagnostic outcomes, consensus results were included in the meta-analysis. In the absence of consensus, we calculated the average sensitivity and specificity and, accordingly, derived the true-positive, false-positive, true-negative, and false-negative values.
Statistical Analysis
For the diagnostic test accuracy meta-analysis, we exported the 2 × 2 contingency table from RevMan for quantitative analysis using suitable models. We applied the bivariate random-effects meta-analysis model to generate summary receiver operating characteristic (SROC) curves.14 Given the presence of zero counts in false-negatives and false-positives, in the 2 × 2 contingency table, we applied a continuity-correction technique to calculate sensitivity and specificity.15 The SROC curve, 95% prediction contour, and 95% confidence contour have been computed. We evaluated heterogeneity both visually and through the I2 statistics as proposed by Chen and Benedetti.16 To investigate publication bias within our diagnostic test accuracy meta-analysis, we used the Deeks funnel plot asymmetry test, considering a P value < .10 as indicative of significant bias. The analyses were performed using the MIDAS17 and METADTA18 custom modules in STATA/MP 17 (https://www.stata.com/statamp/).
RESULTS
Study Characteristics
Our systematic review analyzed data from a total of 14 studies with a pooled sample size of 921 individuals, comprising 5 prospective studies19⇓⇓⇓-23 while others enrolled patients retrospectively.6,26⇓⇓⇓⇓⇓⇓-33 The process of study selection is detailed in the PRISMA flow diagram (Fig 1).24 The sex ratio was almost balanced (male/female ratio: 51%). The age distribution of subjects across the studies showed a mean age of 37.79 (SD, 5.69) years. Among studies that reported AVM location, 426 cases (86.4%) were supratentorial and 67 cases (13.6%) were infratentorial. Among studies that reported the Spetzler-Martin grade, 137 of 275 cases (49.8%) were grade 1, sixty-seven (24.3%) were grade 2, fifty-three (19.2%) were grade 3, and the rest of the cases were grade 4 (6%). The average time interval between the MRI study and the reference test was 57.6 days (ranging from 1 to 240 days). All included studies featured patients undergoing radiosurgery. More detailed information and key findings of each study are summarized in the Table.
PRISMA study flow diagram. During our search across 4 online databases, we identified 3220 studies after deduplication. These were initially screened on the basis of their titles and abstracts, leading to the exclusion of 3117 articles. Of the 103 remaining studies, 5 were inaccessible in full text. The comprehensive review of the remaining 98 full texts resulted in the exclusion of 84 studies due to no comparison between MRI and DSA (72 studies), not enough information for the 2 × 2 contingency table (7 studies), and non-English articles (5 studies).
| Study and Year | Country | Study Design | Population | Mean Ages (yr) | MRI Sequence | Reference Test |
|---|---|---|---|---|---|---|
| Lee et al, 201544 | USA | Retrospective | 136 | 36 | 3D TOF | Biplanar DSA |
| Rojas-Villabona et al, 202121 | Italy | Prospective | 29 | 37 (Range, 18−69) | 4D ASL, 4D MRA | DSA |
| Finitsis et al, 201919 | France | Prospective | 26 | 33 (Range, 22−42) | SWAN | DSA |
| Kodera et al, 201735 | Japan | Retrospective | 7 | 48.7 (Range, 28−80) | ASL | DSA |
| Soize et al, 201422 | France | Prospective | 37 | 45 | 4D MRA | DSA |
| Gauvrit et al, 200620 | France | Prospective | 54 | 40 (Range, 21−72) | 4D MRA | CCA |
| Buis et al, 201230 | Netherlands | Retrospective | 120 | 37.5 (Range, 35.9−39) | 3D TOF | DSA |
| Lee et al, 200926 | Korea | Retrospective | 32 | 33.1 (Range, 12 − 52) | Postcontrast 3D TOF-MRA | CCA |
| Lim et al, 201239 | Korea | Retrospective | 36 | 33.1 SD, 12.9) (Range, 12–61) | 4D MRA | CCA |
| Heit et al, 20206 | USA | Retrospective | 15 | 29.46 (Range, 16–45) | ASL | DSA |
| Leclerc et al, 202029 | France | Retrospective | 28 | 41 | 3D TOF, ASL, SWI, 4D MRA and postcontrast 3D T1 GRE | Biplanar DSA |
| Khandanpour et al, 201323 | UK | Prospective | 23 | 40 | 3D TOF | CCA |
| Pollock et al, 199645 | USA | Retrospective | 164 | Contrast-enhanced T1-weighted | CCA | |
| Quisling et al, 199146 | USA | Retrospective | 34 | Gradient-echo MRI | CCA |
Note:—CCA indicates conventional catheter angiography; GRE, gradient-recalled echo.
Main characteristics of the included studies
Quality Assessment
We used the QUADAS-2 tool (https://www.bristol.ac.uk/population-health-sciences/projects/quadas/quadas-2/) to assess the methodologic quality of the 14 selected studies25 using RevMan. A graphic summary of the quality assessment and more details are presented in the Online Supplemental Data.
Quantitative Synthesis of Diagnostic Performance
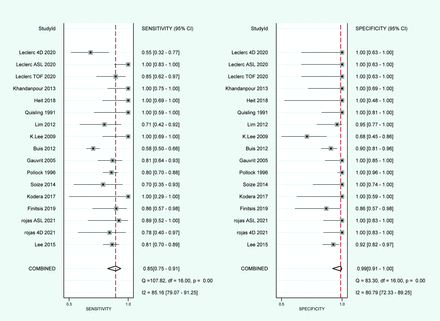
Using the bivariate mixed-effects model, our analysis incorporated data from 14 studies with a total of 921 participants for quantitative synthesis. As represented in Fig 2, the combined sensitivity and specificity of MRI in identifying residual brain AVMs were estimated at 85% (95% CI, 0.75%–0.91%) and 99% (95% CI, 0.91%–1.00%), respectively. The diagnostic odds ratio (DOR) was determined to be 417 (95% CI, 52–3308). Forest plots revealed mild heterogeneity in the DOR (26.1%) but high heterogeneity in sensitivity and specificity (I2 values of 85% and 81%). Figure 3 illustrates the SROC curve with 95% prediction and confidence contours, presenting an area under the curve (AUC) of 97% (95% CI, 90%–99%). On the basis of MR sequences, we performed subgroup analysis in 4 subgroups (Fig 4).
Forest plots of pooled sensitivity and specificity with their 95% CIs.
SROC curve of MRI for the follow-up of brain AVMs after radiosurgery. The summary operating point indicates a sensitivity and specificity of 85% and 99%, respectively, and the AUC is estimated at 0.97.
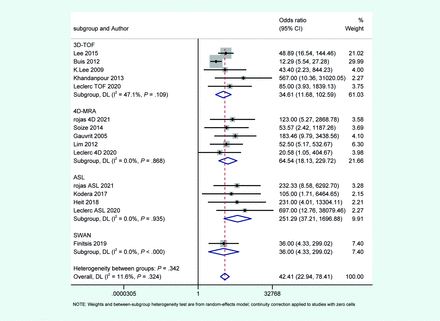
Meta-analysis and subgroup analysis of included articles categorized by MRA type. In the random-effects bivariate-model meta-analysis, the diagnostic accuracy of different MRA sequences was assessed. 3D TOF-MRA showed a pooled sensitivity of 90% (95% CI, 60%–98%) and specificity of 87% (95% CI, 79%–93%) with an AUC of 0.92. 4D MRA had a pooled sensitivity of 72% (95% CI, 62%–81%) and specificity of 99% (95% CI, 92%–100%) with an AUC of 0.97. The ASL sequence had a pooled sensitivity of 90% (95% CI, 78%–96%) and a specificity of 92% (95% CI, 80%–97%) with an AUC of 0.96. The SWAN sequence, evaluated in a single study, reported a sensitivity and specificity of 85.7%.
3D TOF-MRA.
In the random-effects bivariate model meta-analysis comprising 5 included studies evaluating the diagnostic accuracy of 3D TOF-MRA studies, the pooled sensitivity was determined to be 90% (95% CI, 60%–98%), while the pooled specificity stood at 87% (95% CI, 79%–93%). The AUC was calculated to be 0.92.
4D MRA.
In the meta-analysis encompassing all included 4D MRA studies, the pooled sensitivity was determined to be 72% (95% CI, 60%–81%), while the pooled specificity stood at 99% (95% CI, 91%–100%). The AUC was calculated to be 0.97.
ASL.
For the ASL sequence, the pooled sensitivity was determined to be 90% (95% CI, 78%–96%), while the pooled specificity stood at 92% (95% CI, 80%–97%). The AUC was calculated to be 0.96.
SWAN.
We have just 1 article that assessed SWAN for monitoring AVM following radiosurgery21 thus we could not calculate the pooled sensitivity and specificity for this sequence. However, specificity and sensitivity for this method were both reported as 85.7%.
Publication Bias and Sensitivity Analysis
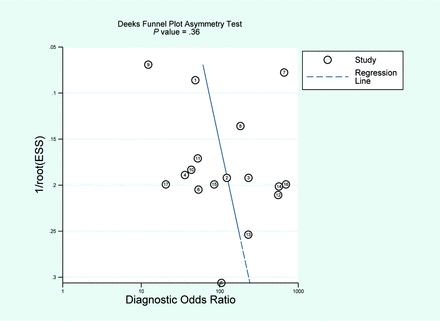
We constructed a Deeks funnel plot to assess the presence of publication bias (Fig 5). The coefficient of the regression line yielded a P value of .36 (P > .05), suggesting that no considerable evidence of publication bias existed.
The Deeks funnel plot was used to investigate the presence of publication bias. The P value suggests that there is no evidence of publication bias (P = .36). ESS indicates effective sample size.
Heterogeneity Analysis
As previously noted, the I2 values for sensitivity and specificity were 85% and 81%, respectively, indicating considerable heterogeneity, but in the DOR, the heterogeneity was 26.1%. Despite variations in sensitivity and specificity, the combined metric of the DOR was relatively consistent across studies. This finding could suggest that even though studies vary in their sensitivity and specificity, the overall DOR is more stable.
To explore the sources of this heterogeneity, we conducted meta-regression analyses using several potential covariates, including MRA type, MRI magnetic field strength, study design (prospective or retrospective), and standard test (DSA and conventional angiography). Follow-up timing and patient populations could potentially contribute to the heterogeneity in our study, but we were unable to analyze these factors due to insufficient data. The findings indicated that there were no significant differences in the OR for these specific covariates. Subgroup analysis results based on MRI type are shown in Fig 4.
DISCUSSION
DSA is considered a criterion standard in AVM assessment, with advantages such as high spatial and temporal resolution and high interobserver agreement. Vascular injury, contrast nephrotoxicity, allergic reaction to contrast media, exposure to ionizing radiation, and neurologic complications are disadvantages of DSA that make the MRI techniques more favorable in assessing AVMs.26 This systematic review and meta-analysis of 14 studies totaling 921 participants found that the MRI pooled sensitivity and specificity in detecting residual brain AVMs were 85% and 99%, respectively. In this regard, Zhou et al27 reported the diagnostic accuracy of MRI for follow-up evaluation of treated AVMs, with a sensitivity of 77% and specificity of 97%. The sensitivity and specificity are both lower compared with our pooled data for brain AVM residuals in follow-up MRI after radiosurgery. Different sequences of MRI are used to image the blood vessels perfectly such as 3D TOF-MRA, ASL, and 4D MRA. Our findings indicate that while different techniques have varying levels of sensitivity and specificity, all 3 demonstrate excellent diagnostic accuracy (AUC = 0.92, 0.96, and 0.97, respectively).
TOF-MRA has become an interesting method to visualize flow within vessels, without requiring contrast media; nevertheless, postcontrast TOF-MRA has superior performance for peripheral blood vessels. Both 2D and 3D TOF methods are used for the imaging of blood vessels. The use of the 2D approach is particularly advantageous in cervical MRA settings, attributable to its broader anatomic coverage and superior vessel-to-background contrast characteristics. Conversely, the 3D TOF technique proves more advantageous for imaging the circle of Willis, primarily due to its enhanced resilience against signal attenuation in the presence of significant vascular stenosis in the TOF-MRA subgroup. However, its effectiveness in visualizing vessels distal to the circle of Willis is somewhat uncertain.33 A study indicated a notable concordance between 3D TOF-MRA and DSA methodologies in discriminating AVFs.28 Notably, the highest level of intermodality agreement relates to AVF size assessment, followed by venous drainage patterns and identification of arterial feeders. Furthermore, comparative analysis revealed superior agreement within 3D TOF-MRA in delineating primary arterial feeders within dural AVFs in contrast to 4D MRA.28 We detected the overall pooled sensitivity, specificity, and AUC of TOF-MRA as follows: 90%, 87%, and 0.92, respectively. Three of our studies used TOF-MRA with 3T machines,23,26,29 while one used a 1.5T system.30 The 1.5T system achieved a sensitivity and specificity of 58% and 89%, respectively, compared with the pooled sensitivity and specificity of the TOF-MRA subgroup, which were 90% and 87%. This difference in the sensitivity could be interpreted in the context of higher SNR and spatial resolution in the 3T system.
In the 3T scanner, the T1 relaxation time is higher and accommodates more background suppression, which eventually results in inflow enhancement and a better contrast-to-noise ratio. In addition, using a short TE (3.5 ms) and multislab technique in the 3T TOF-MRA protocol minimizes the phase dispersion originating from slow flow and results in higher spatial resolution.28,31,32 The TOF-MRA technique is susceptible to artifacts resulting from horizontally directed flow saturation or tortuous vessel segments.33
Using contrast in TOF-MRA could mitigate signal loss resulting from slow or turbulent flow, thereby improving the visualization of peripheral branches and veins emanating from an AVM. However, it might also have negative effects on the study by enhancing adjacent tissues or venous structures.34 In one of the studies within the TOF-MRA subgroup, conducted after contrast injection, a sensitivity of 90%–100% and specificity of 68% were reported.26 The lower specificity observed compared with the pooled specificity of the TOF-MRA subgroup might be attributed to the aforementioned negative impact of enhancing adjacent tissues, though the sensitivity is somewhat higher.
In the ASL subgroup, the pooled sensitivity, specificity, and the AUC from 4 studies are 90%, 92%, and 0.96, respectively. Within this group, 3 used pseudocontinuous ASL,6,29,35 while the remaining used 4D ASL.21
Togao et al36 compared two 4D ASL techniques (CINEMA and 4D-PACK) for visualization of distal cerebral arteries and leptomeningeal anastomosis in patients with Moyamoya disease and found that 4D-PACK shows better visualization and contrast-to-noise ratio. Regarding this finding, 4D-PACK may improve the sensitivity and specificity of 4D ASL in the evaluation of brain AVMs, although the study by Rojas-Villabona et al21 already used CINEMA technique and achieved 100% specificity.
Iryo et al37 performed 4D ASL using CINEMA for the evaluation of intermodality agreement with DSA in dural AVFs, and they found excellent intermodality agreement (κ = 1) in defining the fistula site and venous drainage and good agreement (κ = 0.8) in arterial feeder detection.
Acceleration-selective ASL is another technique that is not influenced by the inflow effect, and it may be able to visualize non-craniocaudal-directed flow as well as slow flows.36 Togao et al38 performed a study to compare the acceleration-selective ASL, TOF-MRI, and DSA in the visualization of brain AVMs. They found that acceleration-selective ASL visualized the feeding artery, nidus, and draining vein of AVMs better than TOF-MRI. The intermodality agreement between ASL and DSA was almost perfect in their study.
4D MRA, also called time-resolved contrast-enhanced MR angiography, using the keyhole method, involves a dynamic acquisition with contrast-enhanced robust timing angiography. Using various spatial and temporal frequency undersampling strategies, it effectively reduces acquisition time without compromising spatial resolution significantly.23,31 It is a promising imaging technique, characterized by its discriminating temporal and spatial resolution, facilitating comprehensive visualization of intracranial circulation across all phases. This capability notably enhances the differentiation of arterial feeders and draining veins. However, there is the potential impact of postcontrast-enhancement ambiguity, especially due to changes that occur after radiosurgery.30 Within our examined studies, 4 investigations used 4D MRA with contrast, with 3 using 3T machines21,22,39 while one used a 1.5T system.20 The sensitivity and specificity derived from the 1.5T study was 81% and 100%, respectively, which is similar to this subgroup's pooled sensitivity and specificity.
Zhuo et al27 performed a systematic review and meta-analysis to assess the effectiveness of contrast-enhanced MRA for detection of residual AVMs in the follow-up of treated brain AVMs. They reported a sensitivity of 77% and a specificity of 97%, along with an AUC of 0.89. These findings are in agreement with our measured sensitivity, specificity, and AUC for 4D MRA of 72%, 99%, and 0.97, respectively. Although the studies included by Zhuo et al were more heterogeneous than our study, our research focused on studies with radiosurgery follow-up and used only contrast-enhanced 4D MRA in the subgroup analysis. 4D MRA shows 100% agreement with DSA in classifying the AVMs on the basis of the Spetzler-Martin grading.40
In our systematic search, only 1 study with the SWAN technique was included, reporting 85.7% sensitivity and specificity with a good intermodality agreement with DSA.19 Hodel et al41 reported a sensitivity and specificity of 87% and 97% for SWI, respectively, with a study population 4 times larger than the previously mentioned study. However, due to the heterogeneity of the treatment procedures, we did not include their results in our analysis. Miyasaka et al42 have reported that SWI is more useful than TOF in depicting the draining veins of AVMs.
Recent advancements in integrating multiple techniques have led to a focus on reducing invasive diagnostic imaging and increasing reliance on non-invasive diagnostic tools, especially for follow-up evaluation. Hodel et al41 studied 92 patients with AVMs after endovascular/radiosurgery treatment. They indicated that using TOF-MRA, 3D MRA, and 4D MRA together resulted in sensitivity, specificity, and AUC values of 90%, 97%, and 0.93, respectively. This combined sensitivity was higher than that of TOF-MRA or 4D MRA alone. In addition, they reported the combined ASL/SWI method having a sensitivity, specificity, and AUC of 98%, 97%, and 0.97 respectively.41
Radiosurgery can result in acute, subacute, and delayed complications, including post-irradiative edema, whose severity depends on factors such as dosage and irradiated tissue volume. Postradiation changes are variable and unpredictable, influenced by tissue type, lesion location, and individual susceptibility to radiation effects. These changes can lead to challenges in distinguishing residual arteriovenous malformation and radiation-induced effects, exacerbated by radiation-induced contrast enhancement. This ambiguity complicates assessments for specific patients and may involve inflammation-induced damage to the BBB, capillary vessel expansion, or the formation of new blood vessels and neoangiogenetic nodules within the perinidal cavity.29
In addition to the noninvasive approach of MRI, noncontrast techniques such as ASL show satisfactory diagnostic accuracy. This result decreases dependence on contrast agents, reducing risks like gadolinium deposition in brain tissue and leading to reduced costs.
ASL offers comprehensive hemodynamic data without necessitating contrast agents, albeit it presents challenges in both acquisition and interpretation. Generally, experience with this application of ASL is limited and involves a steep learning curve. However, the noncontrast nature of ASL avoids confusion induced by postradiation changes derived from radiosurgery that may confuse the radiologist for the evaluation of AVM obliteration.43 The pooled sensitivity, specificity, and AUC for the ASL subgroup were 90%, 92%, and 0.96, respectively, indicating that ASL, together with 4D MRA, has the highest diagnostic accuracy among all subgroups. Additionally, studies that combined ASL with 4D MRA19 and TOF29achieved high diagnostic accuracy. This finding suggests that ASL could be a suitable alternative for follow-up evaluation of brain AVMs after radiosurgery, although it is speculated that using 4D ASL methods and combining them with other sequences such as 4D MRA, SWI, or TOF may augment diagnostic accuracy.
Implementing MRI sequences as a standard monitoring tool poses practical challenges. ASL, for instance, is hindered by both acquisition and interpretation complexities. Limited experience with ASL and its steep learning curve, along with challenges like availability on 1.5T or 3T MRI machines, highlight barriers to widespread adoption. Similarly, accessibility issues with 4D MRA across different MRI facilities worldwide add further obstacles to establishing MRI sequences as standard in monitoring.
Strengths and Limitations
Our meta-analysis provides a comprehensive summary by synthesizing data from 14 studies with 921 participants. We included more studies and sequences than previous reviews, ensuring homogeneous data regarding treatment type and imaging technique. However, high heterogeneity in sensitivity and specificity (I2 > 50%) and the retrospective nature of most studies could introduce bias. Among subgroups, TOF, ASL, and 4D were analyzed by an acceptable number of articles; however, the SWAN method was only assessed in one of our included studies. Like other diagnostic test accuracy meta-analyses, most of the studies in this review evaluated small groups of participants and used different MR techniques and sequences. This variation suggests that the outcomes of this review should be interpreted with caution. Moreover, most studies included in our analysis were retrospective, which could introduce a bias into our search.
CONCLUSIONS
MRI is confirmed as a useful noninvasive imaging technique for AVM follow-up, showing satisfactory sensitivity and specificity compared with DSA. ASL demonstrated the highest pooled sensitivity, while contrast-enhanced 4D MRA showed the highest specificity. MRI allows frequent scans across a patient’s lifetime, offering comprehensive insights into brain changes post radiosurgery. Further studies are needed to establish MRI as the standard over DSA.
Footnotes
Shahriar Kolahi, and Mohammadreza Tahamtan contributed equally to this manuscript.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received May 21, 2024.
- Accepted after revision July 14, 2024.
- © 2025 by American Journal of Neuroradiology