Abstract
BACKGROUND AND PURPOSE: There is a paucity of data on long-term neuroimaging findings from individuals who have developed the post-coronavirus 2019 (COVID-19) condition. Only 2 studies have investigated the correlations between cognitive assessment results and structural MR imaging in this population. This study aimed to elucidate the long-term cognitive outcomes of participants with the post-COVID-19 condition and to correlate these cognitive findings with structural MR imaging data in the post-COVID-19 condition.
MATERIALS AND METHODS: A cohort of 53 participants with the post-COVID-19 condition underwent 3T brain MR imaging with T1 and FLAIR sequences obtained a median of 1.8 years after Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2) infection. A comprehensive neuropsychological battery was used to assess several cognitive domains in the same individuals. Correlations between cognitive domains and whole-brain voxel-based morphometry were performed. Different ROIs from FreeSurfer were used to perform the same correlations with other neuroimaging features.
RESULTS: According to the Frascati criteria, more than one-half of the participants had deficits in the attentional (55%, n = 29) and executive (59%, n = 31) domains, while 40% (n = 21) had impairment in the memory domain. Only 1 participant (1.89%) showed problems in the visuospatial and visuoconstructive domains. We observed that reduced cortical thickness in the left parahippocampal region (t(48) = 2.28, P = .03) and the right caudal-middle-frontal region (t(48) = 2.20, P = .03) was positively correlated with the memory domain.
CONCLUSIONS: Our findings suggest that cognitive impairment in individuals with the post-COVID-19 condition is associated with long-term alterations in the structure of the brain. These macrostructural changes may provide insight into the nature of cognitive symptoms.
ABBREVIATIONS:
- BMI
- body mass index
- COVID-19
- coronavirus disease 2019
- PCC
- post-COVID-19 condition
- reproa
- Reproducibility Analysis
- SARS-CoV-2
- Severe Acute Respiratory Syndrome coronavirus 2
- WAIS
- Wechsler Adult Intelligence Scale
- WHO
- World Health Organization
Post-COVID-19 condition (PCC) refers to the persistent and multisystemic symptoms that some individuals develop after infection with Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2), even if they experienced an asymptomatic or mild coronavirus 2019 (COVID-19). Recent studies indicate that 5% and 30% of COVID-19 survivors of the first, second, and third waves and about 65% of those hospitalized due to the disease may have the PCC.1⇓-3 According to the World Health Organization (WHO) criteria, the PCC occurs in individuals with a history of probable or confirmed SARS CoV-2 infection, usually 3 months after the onset of COVID-19 with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis.4 Common symptoms include fatigue, shortness of breath, and cognitive dysfunction, all of which negatively impact daily functioning. After initial recovery from an acute COVID-19 episode, symptoms may manifest for the first time or persist from the initial illness. In addition, symptoms may fluctuate or relapse across time.5
According to a recent study, previous COVID-19 infection might have caused at least 1 incident condition in 20% of the survivors 18–64 years of age and in 25% of the survivors 65 years of age or older.6 This finding significantly impacts their quality of life. Various mechanisms have been speculated to contribute to the impairment of the CNS. Different pathophysiologic mechanisms have been proposed to explain these changes.6,7
Several studies have documented the cognitive impact of SARS-CoV-2 infection, but the underlying brain changes are still unclear.8 Few studies have performed a comprehensive neuropsychologic assessment in patients recovered from COVID-19 with objective cognitive symptoms; this assessment could provide valuable insight into the cognitive impact of the infection. The most common cognitive impairments objectified by COVID-19 with the PCC are in attention, memory, and executive functions.8,10⇓⇓⇓-14
Few studies have performed associations between brain alterations and cognitive deficits.8,15-16 Furthermore, there is a paucity of data on the long-term neuroimaging findings related to cognitive function in individuals who develop the PCC; this information could shed light on the pathophysiology and prognosis of these patients. The structural changes in GM volume, which may explain these cognitive deficits, are inconsistent across studies. Some studies have reported reduced GM volume in the hippocampus after a mean of 11 months postinfection compared with noninfected controls.8,15 The hippocampus is involved in memory formation and consolidation.17⇓⇓-20 Another study found that patients with the PCC with fatigue had lower GM volume in the left thalamus, putamen, and pallidum, which is related to attention and short-term memory.21 On the other hand, 1 study observed an increased GM volume in the thalamus and the hippocampus, but a decrease in the cortical regions in patients with the PCC.22 None of these studies evaluated associations between MR imaging findings and the cognitive assessment outcomes. Other studies found no significant difference in GM volume between patients having acqired COVID-19 and noninfected controls having acquired SARS-CoV-2. Those studies were conducted between 2 and 11 months after SARS-CoV-2 infection.8,23⇓⇓-26 No studies have been conducted nearly 2 years after the first SARS-CoV-2 infection in patients with persistent cognitive symptoms.
The aim of the present study was to elucidate the long-term cognitive outcomes of participants with the PCC.
MATERIALS AND METHODS
Standard Protocol Approvals, Registrations, and Patient Consents
The research project followed the ethics standards and guidelines of the ethics committee of the Foundation University Institute for Primary Health Care Research Jordi Gol i Gurina, which approved the study protocol (reference 21/220-P). The participants were informed of the objectives, procedures, risks, and benefits of the study, and they provided written consent to participate and share their data for research purposes. The data were pseudoanonymized using a numeric coding system and stored securely in a REDcap database (https://www.project-redcap.org/). All procedures were conducted according to good clinical practices and the General Data Protection Regulation 2016/679 on data protection and privacy for all individuals within the European Union.
Study Population
The Alliança ProHEpiC Cognitiu study (The APC Study) aims to investigate the cognitive and neural effects of COVID-19 infection in different groups of individuals. The study includes participants who have persistent symptoms after recovering from COVID-19 with and without cognitive symptoms, as well as participants who were infected but did not develop the PCC and uninfected paricipants.27 In this report, we present the structural MR imaging data of a subsample that underwent brain imaging and cognitive assessment as part of the study.
This cross-sectional study involved 53 participants who had the PCC with cognitive symptoms after recovering from COVID-19. They were recruited from primary health centers and hospitals in Northern Barcelona (Spain) between August 1, 2020, and March 2023. The study followed the WHO criteria for confirming the PCC diagnosis.4 The participants also had to be at least 12 weeks postinfection and between 20 and 70 years of age. The study excluded those with pre-existing psychiatric, neurologic, or neurodevelopmental disorders that could cause cognitive deficits, those with a history of drug or alcohol abuse or a life expectancy of <6 months, and those who could not undergo MR imaging due to medical contraindication or claustrophobia.
Study Procedure
The study collected data from the participants in 2 sessions. In the first session, participants provided sociodemographic information: sex (male, female), age (in years), education level (elementary, secondary, high school, university degree, specialist or master, doctorate), job field (doctor, nurse, health services, health assistants, other), weight (kilograms), height (centimeters), body mass index (BMI) (according to the WHO standards):28 underweight, normal weight, overweight, obesity class I, obesity class II, obesity class III), high blood pressure (Yes, No), cholesterol (Yes, No), diabetes (Yes, No), tobacco (never, smoker, ex-smoker), and alcohol consumption (Yes, No). During this first session, all participants completed a comprehensive neuropsychological assessment (see below). They also reported their COVID-19 experience (date and method of diagnosis, clinical spectrum including symptoms and treatment). In the second session, individuals with a PCC underwent a brain MR imaging within 6 months from the cognitive assessment. To protect the privacy of our participants, we used cryptographic hashtags to anonymize the project database. We also used the same 10-digit numeric encoding system hosted by REDCap, Version 12.4.22, Vanderbilt University, for the MR imaging study.
Cognitive Assessment
The cognitive domains of executive function, attention and processing speed, memory, language, and visuospatial and visuoconstructive functions were evaluated by a comprehensive neuropsychological battery administered by a trained, qualified clinical neuropsychologist with >5 years of experience in the assessment of neurologic disorders and a psychologist carefully trained and supervised by the same clinical neuropsychologist. The cognitive tests used for each domain were as follows: for executive functions, the Digit Span Backward subtest from the Wechsler Adult Intelligence Scale 3rd edition (WAIS-III),29,30 (a difference score [B-A] that removed the speed element from the test evaluation was calculated);31 the phonetic (letters beginning with P, M, and R, 1 minute each) and semantic verbal fluency tests (“animals” in 1 minute);32,33 and the interference score of the Color-Word Stroop Test;34 for attention and velocity, the Digit Span Forward subtest,29,30 the Symbol Search from the WAIS-III,35 the Trail-Making Test-A,29,30 and the Symbol Digit Technique Test (WAIS-III);32 for memory, the total learning and delayed recall from the Rey Auditory Verbal Learning Test36 and the delayed recall from the Rey Osterrieth complex figure (ROCF);37,38 for language, the short version of the Boston Naming Test39 and the vocabulary test from the WAIS-III;35 and for visuospatial and visuoconstructive functions, the copy accuracy of the ROCF.37,38 Fatigue was assessed with the Modified Impact Fatigue Scale. This scale includes 3 subscales: cognitive, physical, and psychosocial. In this scale, participants are asked to rate the extent of fatigue in their life in the past 4 weeks, with 0 indicating no problem and 4 indicating extreme problem. There are a total of 21 items, 10 cognitive items, 9 physical items, and 2 psychosocial items. The maximum score is 76, forty for the cognitive subscale, 28 for the physical subscale, and 8 for the psychosocial scale, with a score higher than 38 meaning significant fatigue.40
Criteria for Cognitive Impairment
We used the Frascati criteria41 to assess cognitive impairment in participants with the PCC because there is no widely accepted standard for this population. According to these criteria, patients have cognitive impairment if they score below −1.5 SD on any subtest within a cognitive domain or below −1 SD on 2 subtests of the same cognitive domain.
Neuroimaging
Neuroradiologic Assessment of the Structural MRIs.
The assessment was conducted by the neuroradiologist, who has 21 years of expertise, primarily aimed at excluding the presence of brain lesions. This exclusion was achieved through the examination of T1-weighted, FLAIR, and diffusion sequences, with a specific focus on identifying regions of encephalomalacia due to trauma or previous surgical interventions, territorial vascular infarctions, lacunes, or brain tumors. No incidental findings were identified that needed specific medical attention or further secondary testing in our cohort.
A total of 5 individuals had isolated and nonspecific hyperintensity in the supratentorial, subcortical WM. These hyperintensities did not correspond to lacunar infarcts and were not considered for the analysis of cerebral cortical thickness.
A clinical neuroradiologist performed an initial assessment to ensure that none of the 53 participants showed ischemic pathology or other macrostructural injuries.
MR Imaging Acquisition Protocol.
All images were acquired on a Vantage Galan 3T MR imaging (Canon Medical Systems) at the Center for Comparative Medicine and Bioimage Image (Germans Trias i Pujol Research Institute, Badalona, Spain) using a 32-channel head SPEEDER coil (AcanMed) with foam padding and headphones to limit head movement and suppress scanner noise. The MR imaging protocol included a 3D MPRAGE T1-weighted sequence acquired in the sagittal plane (TR = 2000 ms; TE = 2.7 ms; 158 slices; section thickness = 1 mm; no gap; matrix = 256 × 256; in-plane resolution = 1 × 1; TI = 900 ms; flip angle = 15°; FOV = 256 × 256 mm; and voxel size = 1 × 1 × 1 mm3; seven minutes and 50 seconds), and a FLAIR image, also in the sagittal plane (TR = 5000 ms; TE = 504 ms; 72 slices; section thickness = 1 mm; no gap; matrix = 256 × 256; in-plane resolution = 1 × 1; T1 = 1500 ms; flip angle = 15°; FOV = 256 × 256 mm; voxel size = 1 × 1 × 1 mm3; five minutes and 15 seconds).
MR Imaging Analysis
FreeSurfer Analysis.
We used FreeSurfer, Version 7.3.2 (http://surfer.nmr.mgh.harvard.edu) to analyze MR imaging data. This software reconstructs gray/white matter and pial surfaces and measures cortical thickness based on intensity and continuity information from MR imaging volumes. It also segments the whole brain and measures subcortical volumes.42 We used T1 and FLAIR images for FreeSurfer parcellation, which improve its reliability and robustness. We still had issues with the skull-stripping for some participants, which has been amended using mri_gcut (https://surfer.nmr.mgh.harvard.edu/fswiki/mri_gcut) in FreeSurfer. We also visually inspected the final parcellations for all participants and found no error or artifactual parcellation. We focused our analyses on 46 anatomic ROIs (Online Supplemental Data).
GM and WM Preprocessing Protocol.
We used Reproducibility Analysis (reproa, https://github.com/reprostat/reproanalysis), a neuroimaging pipeline written in Matlab (MathWorks) to process MR imaging data, which commands SPM12 (Statistical Parametric Mapping; https://www.fil.ion.ucl.ac.uk/spm/) functions. We first aligned the 3D T1 image to the Montreal Neurological Institute template and then aligned the FLAIR image to the previously registered 3D T1 image using a 6 df linear transformation. We applied multichannel segmentation (SPM12, “Unified Segmentation and Normalization”) to the aligned 3D T1 and FLAIR images to obtain 6 tissue classes: GM volume, WM volume, CSF, bone, soft tissue, and residual noise. We used Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra Toolbox (DARTEL, part of SPM) to register the GM and WM images from native space to a custom standard space. We then normalized the group template to the Montreal Neurological Institute template using an affine transformation and applied the combined normalization parameters to each participant’s GM and WM images, preserving density estimates. For voxel-based morphometry analysis, we scaled the normalized GM and WM images by the total tissue volume and smoothed them with an 8-mm full width at half maximum Gaussian kernel. The estimated GM and WM templates were further corrected for intracranial volume.
Statistical Analysis.
Statistical analyses on demographic data were performed with the Statistical Package for the Social Sciences, Version 17.0 for Windows (SPSS; IBM). The distributions of demographic variables were tested for normality using the Shapiro-Wilk test. Continuous variables were expressed as mean (SD) or median and interquartile range. Categoric variables were defined as frequencies and percentages. Cognitive tests were z scored before the analysis, considering age and years of education for the main tests. We used the model comparison to test whether a linear model including the BMI could explain individual variation in global GM.
We extracted the volumes and cortical thickness of 46 regions from the FreeSurfer output (Online Supplemental Data), and we normalized them by total intracranial volume. We then performed multiple linear regression analyses to test the relationship between the normalized volumes and the cognitive performance for each cognitive domain. We controlled for sex, age, and years of education. We also tested whether including the BMI improved the model. We corrected for multiple testing using a false discovery rate at a threshold of q = 0.05, corresponding to a significance level of P = .033.
To examine the association between GM volume and WM volume and cognitive domains, we used the Randomize tool (Version 2.1; www.fmrib.ox.ac.uk/fsl/randomize/index.html) from the FMRIB Software Library (FSL; http://www.fmrib.ox.ac.uk/fsl), which performs permutation tests on voxel-based morphometry data.43 We set the permutations to 10,000 and applied the tool to the GM and WM templates generated by reproa.44 We constructed a general linear model to identify the brain regions in which GM and WM volumes were significantly related to the cognitive domains. We applied threshold-free cluster enhancement to correct for multiple comparisons. We also included age, sex, and years of education as covariates in the model.
RESULTS
Demographic and Clinical Characteristics
The results of the demographic and clinical characteristics are summarized in the Online Supplemental Data. The sample consisted of 53 individuals mainly with a history of mild COVID-19 infection, with a mean age of 48.23 (SD, 9.2) years and a mean education level of 14.04 (SD, 2.6) years. Most participants were women (88.7%) and had a mild-moderate clinical spectrum of COVID-19 (81.1%). Only 17% of the participants required hospitalization in the acute phase due to COVID-19. The most common vascular risk factors of the sample were smoking (current or former, 44.2%) and alcohol consumption (40.4%). According to the WHO standards, 34% of the participants were overweight (BMI = 25–29.9), 13.2% were obese (BMI 30–34.9), and 13.2% were extremely obese (BMI ≥35). The mean time since the diagnosis of COVID-19 was 1.8 years.
The Online Supplemental Data summarize the self-reported symptoms experienced by participants with the PCC at the time of evaluation. The most common symptoms were weakness (92.5%), discomfort, and fatigue (83%), followed by nonspecific insomnia (80%), muscle pain (71.9%), vertigo and dizziness (71.2%), and tingling sensations (66.7%). The most common cognitive symptoms were difficulty with concentration and memory (96.2%), followed by brain fog (84.9%); 92.5% of the sample scored >38 points on the Modified Impact Fatigue Scale, which measures fatigue, indicating significant fatigue.
Cognitive Characteristics
The results of the neuropsychological tests are summarized in the Online Supplemental Data. We found that cognitive tests showing the most impairment, with scores below −1.5 SD, were semantic verbal fluency (41.5%), phonologic verbal fluency (32.1%), and digit span forward (33%). When we considered cognitive domains and following the Frascati criteria, the cognitive domains showing the most impairment were executive function (58.5%), attention and processing speed (54.7%), and memory (39.6%). Only 1 participant showed problems in the visuospatial and visuoconstructive functions (1.9%). None had difficulty in the language domain.
GM and WM Voxel-Based Morphometry Results (Reproa)
We did not find any significant corrected result in the correlations between GM or WM volume and cognitive symptoms.
Adding the BMI to the regression model led to a nonsignificant increase in the log likelihood (χ2(1) = 1.6569, P = .198), indicating that the BMI does not account for the additional variance of the global GM. Thus, BMI was omitted as a covariate from the general linear model. Also, according to the Akaike and Bayesian information criteria, the model without the BMI was a better fit (Akaike Information Criterion: 1455.55 versus 1455.89 and Bayesian Information Criterion: 1463.43 versus 1465.74 for the model without and with BMI, respectively).
Cortical Thickness (FreeSurfer)
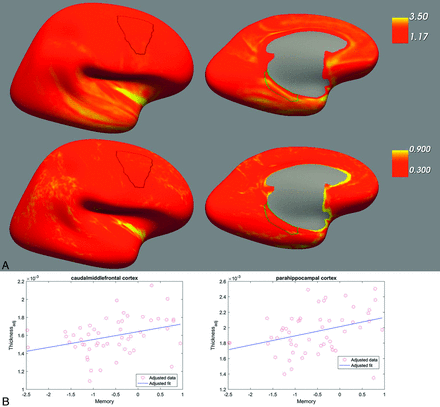
The regression analysis showed that lower memory performance was significantly associated with a decrease of the cortical thickness of 2 brain regions after adjusting for total intracranial volumes, sex and age, ROIs, and the number of cognitive domains tested. The results indicate that 1 SD decrease in memory performance is associated with 0.20-µm and 0.09-µm reductions in the left parahippocampal (adjusted r2 = 0.3, b = 1.20 × 10−4; CIb = 0.14 × 10−4; 2.25 × 10−4, t(48) = 2.28, P = .03) and the right caudal-middle-frontal (adjusted r2 = 0.27, b = 0.87 × 10−4; CIb = 0.08 × 10−4; 1.67 × 10−4, t(48) = 2.20, P = .033) regions, respectively (Figure).
A, FreeSurfer view of the cortical thickness estimates averaged across the whole sample of 53 participants. The left image shows the right caudal-middle frontal region, and the right image shows the left parahippocampal region. The upper row shows the mean estimates, and the lower row shows the SD. B, Relationship between the (adjusted) cortical thickness (in millimeters) and the performance in the memory domain (in z scores) as estimated by the linear regression model.
DISCUSSION
The current study examined the brain structural changes in 53 participants with the PCC who reported cognitive symptoms nearly 2 years after infection, with an average age of 48.23 years and a predominance of women.
We found that the most common symptoms were weakness, discomfort, and fatigue, followed by nonspecific insomnia, muscle pain, vertigo and dizziness, and tingling sensations. The participants’ cognitive symptoms were consistent with those in previously published studies.8,10⇓⇓⇓-14 The cognitive tests showing the most impairment were semantic verbal fluency, phonologic verbal fluency, and digit span forward. The cognitive domains showing the most impairment were executive function, attention and processing speed, and memory.
We found that decreased cortical thickness of the left parahippocampal region and the right caudal-middle-frontal region was positively associated with the impairment of the memory domain. Lower scores in this domain were associated with less cortical thickness in these anatomic regions. The parahippocampal part is the medial temporal lobe, and it has been linked to subjective memory symptoms and very early Alzheimer disease.45,46 Only 4 studies have investigated the long-term effects of COVID-19 on cortical thickness, with conflicting results. Douaud et al15 found that reduced GM thickness in the orbitofrontal cortex and the parahippocampal gyrus was associated with cognitive impairment. Sanabria-Díaz et al47 reported lower cortical volume in the orbitofrontal, frontal, and cingulate regions but did not assess cognitive function. Petersen et al48 found no difference in cortical thickness between patients recovered from COVID-19 versus uninfected matched controls or any association with cognition. Finally, Rothstein22 observed reduced GM thickness in several cortical regions but did not relate this to any cognitive test. It is necessary to investigate the cognitive and brain structural changes associated with the PCC to gain a better understanding of its nature and to develop cognitive therapies to improve patients’ neurocognitive symptoms.
As far as we know, this is the first study to identify macrostructural alterations in a cohort of young and middle-aged individuals almost 2 years after experiencing the PCC, along with cognitive symptoms that persisted and were objectively measured. When we compared these results with the those in the published literature, only 2 studies, conducted by Douaud et al15 and Díez-Cirarda et al,16 identified similar findings in the parahippocampal and hippocampal regions, but in an elderly population (51–81 years of age) in the first one and in 1 young middle-aged cohort (50.89 [SD, 11.25] years) in the second one. We also found statistically significant cortical thickness reduction in the right caudal-middle-frontal region. This region involves age-related memory impairment.49 Douaud et al15 found reduced GM thickness in the orbitofrontal cortex. As in our case, it was linked to memory impairment. On the other hand, other studies found reduced cortical thickness in the orbitofrontal and cingulate regions, but its relationship with cognitive function needed clarification because no associations between the MR imaging findings and cognition were conducted.22,47
The fact that we did not find any significant gray or white matter volume alterations does not rule out the possibility of brain changes, as occurs frequently in patients with early-stage Alzheimer disease.50 The link between SARS-CoV-2 infection and new-onset autoimmune diseases is strong and covers a wide range of disorders,51 including myalgic encephalitis and chronic fatigue syndrome.52 More specifically, patients with chronic fatigue syndrome and myalgic encephalitis have been reported to experience memory and concentration problems and difficulties in processing complex information.53 Consistently, Thapaliya et al54 found cortical thickness reduction in the caudal-middle-frontal region in patients with chronic fatigue syndrome and myalgic encephalitis. Multiple sclerosis has been proposed as a model to study the effects of SARS-CoV-255 as well. In a study conducted by Fujimori et al, in 2021,56 the authors found that cortical thickness reduction patterns in MS are mostly characterized by the degree of temporal lobe cortical atrophy, which may start in the relapsing-remitting phase.
Furthermore, the persistent cognitive problems, such as memory loss, confusion, and difficulty concentrating shown in individuals who have the PCC are similar to those experienced by some patients with cancer after undergoing chemotherapy or radiation therapy, a condition known as cancer-related cognitive impairment or “chemo brain,” which targets the hippocampus as well.57 Unfortunately, the prognosis for those diseases is often poor.58 Last, neurodegenerative diseases with cognitive impairment have been linked to those anatomic regions as well.46
We think that the gray and white matter voxel-based morphometry is hampered by the lack of an uninfected SARS-COV-2 or recovered COVID-19 matched control group, as well as the small sample size.
In addition, we are confident that there must be functional alterations in the brain, because these usually occur before a structural region is affected and account for structural changes.59
Our study is strengthened because we are assessing participants with the PCC and cognitive symptoms 1.8 years after the SARS-CoV-2 infection in a relatively young population, and we found brain structural abnormalities associated with objective cognitive symptoms. These abnormalities could not have been detected with conventional MR imaging within the health care system because we used an exhaustive research-oriented MR imaging protocol.
Although this study is one of the most complete, including neuroimaging and cognitive evaluation of patients with the PCC available to date, it also has several limitations. Larger samples should replicate these findings to generalize them to this population type and to include control groups. Future research should address 3 different lines of research: First, this type of MR imaging analysis should be repeated in individuals with the PCC with cognitive symptoms and should compare them with control groups; second, these same studies should be repeated for longer periods to determine whether these structural changes are sustained across time, evolve, or reverse. Finally, cognitive interventions should be tested to assess whether they have a significant impact on cognition and its neural correlates.
CONCLUSIONS
The present study used a comprehensive neuropsychologic battery and a highly specialized MR imaging protocol to investigate brain volumes and cortical thickness and their associations with cognitive function in 53 relatively young participants. Our results showed that the cognitive deficits were associated with changes in brain macrostructure, especially in the left parahippocampal region and the right caudal-middle-frontal region, possibly explaining the cognitive symptoms described by these participants.
Acknowledgments
The authors would like to sincerely thank the participants for their effort and selfless involvement in the study. In addition, they want to thank the Management Department, Primary Care Directorate, and the Directorate of the Clinical Laboratory of the Metropolitan North for the facilities they have given for the project.
Footnotes
This research was funded by grant No. SLT0020/6_14 and in the call for grants corresponding to the year 2021 of the Strategic Plan for Research and Innovation in Health 2016–2020, modality research projects oriented to primary care, with file code SLT002/000055 of the Departament de Salut. Generalitat de Catalunya. This study is also supported, in part, by grants from National Health Institute Carlos III COV20/00660 to J.G.P. and by the CIBER-Consorcio Centro de Investigación Biomédica en Red (CB 2021), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación and Unión Europea, Next Generation EU. M. Mataró is supported by ICREA Academia program. M. Mataró acknowledge research funding by a “Ramon y Cajal” contract (RYC2020-028934-I/AEI/10.13039/501100011033) from the Spanish Ministry of Science and Innovation.
The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of this work.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received September 19, 2023.
- Accepted after revision January 3, 2024.
- © 2024 by American Journal of Neuroradiology