Abstract
BACKGROUND AND PURPOSE: Reduced cerebral perfusion has been observed with elevated intracranial pressure. We hypothesized that arterial spin-labeled CBF can be used as a marker for symptomatic hydrocephalus.
MATERIALS AND METHODS: We compared baseline arterial spin-labeled CBF in 19 children (median age, 6.5 years; range, 1–17 years) with new posterior fossa brain tumors and clinical signs of intracranial hypertension with arterial spin-labeled CBF in 16 age-matched controls and 4 patients with posterior fossa tumors without ventriculomegaly or signs of intracranial hypertension. Measurements were recorded in the cerebrum at the vertex, deep gray nuclei, and periventricular white matter and were assessed for a relationship to ventricular size. In 16 symptomatic patients, we compared cerebral perfusion before and after alleviation of hydrocephalus.
RESULTS: Patients with uncompensated hydrocephalus had lower arterial spin-labeled CBF than healthy controls for all brain regions interrogated (P < .001). No perfusion difference was seen between asymptomatic patients with posterior fossa tumors and healthy controls (P = 1.000). The median arterial spin-labeled CBF increased after alleviation of obstructive hydrocephalus (P < .002). The distance between the frontal horns inversely correlated with arterial spin-labeled CBF of the cerebrum (P = .036) but not the putamen (P = .156), thalamus (P = .111), or periventricular white matter (P = .121).
CONCLUSIONS: Arterial spin-labeled–CBF was reduced in children with uncompensated hydrocephalus and restored after its alleviation. Arterial spin-labeled–CBF perfusion MR imaging may serve a future role in the neurosurgical evaluation of hydrocephalus, as a potential noninvasive method to follow changes of intracranial pressure with time.
ABBREVIATIONS:
- ASL
- arterial spin-labeled
- ICP
- intracranial pressure
- PF
- posterior fossa
Hydrocephalus is a common neurosurgical condition in children and adults, accounting for approximately 69,000 annual hospital admissions and 39,000 shunt procedures in the United States.1,2 While concepts of hydrocephalus remain complex, including pathophysiology, diagnostic and therapeutic approaches,3⇓–5 and outcome,6,7 it is generally accepted that hydrocephalus reflects pathologic dynamics among brain, spinal cord, blood, and CSF within a confined environment.8⇓–10 In clinical practice, imaging is often used to work-up hydrocephalus in search of obstructing lesions or the presence of ventriculomegaly. However, ventricular size, a frequently used imaging feature, does not always correlate with underlying CSF pressures or resorptive capacity for CSF11⇓⇓⇓⇓–16 and, therefore, may not reliably identify compensated hydrocephalus without signs of raised intracranial pressure (ICP) and progressive hydrocephalus with raised ICP.
Prior studies have shown reduced CBF with elevated ICP in various animal models of hydrocephalus.17⇓⇓–20 Reduced CBF has also been reported in small case series of children with either congenital hydrocephalus or acute hydrocephalus from brain tumors by using 15O-PET21 or SPECT.22 Recently, 2D phase-contrast MRA has shown that carotid and basilar arterial flow rates are reduced in infants with hydrocephalus.23 However, 2D phase-contrast MRA does not directly measure CBF at the tissue level and may be hampered by long scanning times and flow-sensitive technical challenges.24,25
In contrast, arterial spin-labeled (ASL) MR imaging perfusion directly measures tissue perfusion without requiring long scanning times, contrast, or radioisotope injection.26 It is also uniquely advantageous in children with high labeling efficiency and SNR and can be repeated in the event of patient motion or after CSF diversion without the risk of radiation exposure.27 While ASL perfusion has increasingly become clinically available, no studies have investigated its role for evaluating hydrocephalus. We hypothesized that ASL-CBF is reduced in uncompensated hydrocephalus and improved after its alleviation and, therefore, can be used as a marker for symptomatic hydrocephalus.
Materials and Methods
Patients with Symptomatic Hydrocephalus
All patients presenting with a posterior fossa (PF) brain tumor at our children's hospital from 2010 to August 2013 were retrospectively reviewed after approval by the institutional board (protocol 28674). The study cohort was identified by using the following inclusion criteria: The patients underwent a baseline ASL perfusion MR imaging at 3T, presented acutely with clinical signs of elevated ICP that required neurosurgical intervention (tumor resection or CSF diversion) within 7 days of a baseline ASL scan, had ventriculomegaly, and received no prior medical/radiation therapy or neurosurgical intervention, including CSF diversion.
One of the important pathogenic factors in hydrocephalus is increase in resistance to outflow of CSF, which has been correlated with elevated CSF pressure.11,14 Children acutely presenting with uncompensated hydrocephalus from a new PF tumor that required symptom alleviation were selected as our study cohort, given that this group represented a relatively uniform cohort with similar obstructive mechanisms and could be distinguished from those with chronic compensated or uncompensated hydrocephalus.
Patients with underlying cardiac disease, epilepsy, migraines, hemorrhage, vascular lesions (aneurysm, AVM, fistula, or stenoocclusive disease), prior strokes, concomitant supratentorial tumors or leptomeningeal seeding, and prior history of radiation or chemotherapy were excluded, given their potential impact on cerebral perfusion.
Asymptomatic Patients
Patients with an incidentally discovered PF tumor but without signs of elevated ICP based on neurosurgical assessment and without ventriculomegaly or interstitial periventricular edema on MR imaging were also included for comparison if they had undergone a treatment-naïve ASL perfusion MR imaging at 3T.
Controls
Healthy controls who underwent ASL perfusion at 3T as part of routine brain MR imaging were randomly selected from our data base and matched for age and sedation status of the study subjects. The control group consisted of children with no underlying neurologic diseases and normal brain MR imaging findings. Examples of clinical reasons for undergoing MR imaging were isolated headaches, cholesteatoma of the middle ear, isolated facial lesions (eg, hemangioma, dermoid), scalp nevus, inflammatory nasal obstruction, short stature, and family history of aneurysms. Children with neurocutaneous syndromes; ataxia; gaze abnormality; developmental delay; endocrinopathies; psychiatric diseases; migraines; and any chronic diseases, including epilepsy, cancer, and previously treated neurologic diseases; and prior or ongoing medical therapy were excluded.
Clinical Assessment
Neurosurgical documentation regarding patient symptoms at baseline and after neurosurgical intervention was reviewed, including the patient's clinical status at follow-up ASL perfusion and, if present, the functional status of the shunt or ventriculostomy.
Imaging Methods
All subjects underwent brain MR imaging at 3T (Discovery 750; GE Healthcare, Milwaukee, Wisconsin), by using an 8-channel head coil. The technique used to perform perfusion ASL MR imaging has been described in detail elsewhere.28 Briefly, our vendor-supplied ASL was performed by using a pseudocontinuous labeling period of 1500 ms, followed by a 1500-ms postlabel delay. Whole-brain images were acquired with a 3D background-suppressed fast spin-echo stack-of-spirals method, with a TR of approximately 5 seconds. Multiarm spiral imaging was used, with 8 arms and 512 points acquired on each arm (bandwidth, 62.5 kHz), yielding in-plane and through-plane spatial resolutions of 3 and 4 mm, respectively. A high level of background suppression was achieved by 4 separate inversion pulses spaced around the pseudocontinuous labeling pulse. The sequence required 5 minutes to acquire, which included proton-attenuation images required for CBF quantitation. For graphic prescription of the ASL, the sagittal image following the 3-plane localizer was used for alignment. Postprocessing was performed by using an automated reconstruction script that returned CBF images directly to the scanner console within 1 minute, by using the microsphere methodology described by Buxton et al.29 Other ASL MR imaging parameters were TR/TE, 4632/10.5 ms; FOV, 24 cm; matrix, 512 × 8; and NEX, 3.
Imaging Analysis
ASL Perfusion.
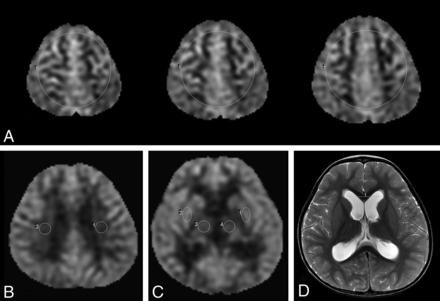
Quantitative mean cerebral CBF was measured and averaged at 3 consecutive axial images just above the centrum semiovale near the vertex by using region-of-interest analysis (size, 6500 mm2). The axial planes near the vertex were selected, given a higher proportion of gray matter in this region, and because higher SNR is seen in the gray matter compared with white matter.30,31 Also, a large region-of-interest size was chosen in this region for a more global CBF assessment that is easy to perform in a clinical setting by a radiologist or a neurosurgeon. In addition, more specific anatomic regions were interrogated by using region of interest (size, 85 mm2) at the bilateral basal ganglia (at the level of putamina), thalami, and periventricular white matter. Examples are shown in Fig 1. The ROIs were independently performed by 2 neurosurgeons (A.A., R.M.L.) blinded to clinical information, including the status of hydrocephalus and follow-up clinical management. A blinded pediatric neuroradiologist (K.W.Y.) with a Certificate of Added Qualification in neuroradiology (7 years of experience) confirmed the proper region-of-interest placement.
Brain region-of-interest placement and ventricular measurement. A, ROIs were placed in the 3 axial levels of the cerebrum near the vertex and just above the centrum semiovale. B and C. Additional ROIs were placed in bilateral periventricular white matter, and the deep gray matter, including the bilateral thalami and putamina. D, Ventricular measurement of the bilateral frontal horns was performed at the level of the caudate heads.
For comparison between CBF at baseline and after neurosurgical intervention, the last follow-up ASL perfusion scan at 3T before initiation of any medical/radiation therapy was used so that no therapy other than tumor decompression or CSF diversion occurred in the interval between the baseline and the follow-up MR imaging.
Ventricular Size and Edema
The bifrontal diameter at the level of the caudate heads and underlying perfusion were measured in each patient at baseline and serial follow-up MR imaging by a neuroradiologist (K.W.Y.) blinded to patient clinical status (symptomatic versus asymptomatic with regard to hydrocephalus) at the time of imaging. The presence or lack of periventricular interstitial edema was noted based on T2-weighted images.
Statistical Analysis
All statistical analyses were performed with the Statistical Package for the Social Sciences, Version 20.0 (IBM, Armonk, New York) with an a priori significance level of α =.05. Associations of perfusion at various sites (eg, thalamus, putamen, and white matter) with age and diameter between the frontal ventricular horns were tested by using the 2-tailed Pearson correlation. Comparisons of perfusion between sexes and between patients with and without interstitial edema were made by using the Mann-Whitney U test for independent samples. Comparisons of perfusion between healthy controls and asymptomatic patients with tumor and those symptomatic from obstructive hydrocephalus, based on tumor type, were made by using the Kruskal-Wallis test for independent samples with pair-wise comparisons. Differences between perfusion before and after relief of obstructive hydrocephalus were evaluated with the Wilcoxon signed rank test for related samples.
Results
Clinical Findings
Twenty-three patients, median age, 6.2 years (range, 0.9–18 years), were included in the study. Patient demographics and clinical data are shown in Table 1. The control group matched by age and sedation status consisted of 16 children (7 boys and 9 girls; median age, 8.2 years; range, 1–18 years).
Patient characteristics (n = 23)
Patients with Symptomatic Hydrocephalus
Nineteen patients with PF tumor presented with symptomatic obstructive hydrocephalus, 16 of whom underwent at least partial resection. In 6 of these patients, CSF diversion in the form of an extraventricular drain or ventriculoperitoneal shunt was performed at the time of the surgery or subsequent to tumor resection. Three patients with unresectable brainstem tumors also underwent CSF diversion: ventriculoperitoneal shunt placement in 1 case of diffuse intrinsic pontine glioma and endoscopic third ventriculostomy in 2 cases of tectal astrocytoma.
Seventeen patients demonstrated symptomatic improvement after removal of the obstructing mass and/or CSF diversion. Two patients continued to demonstrate intractable hydrocephalus after tumor resection either from shunt complications or extraventricular drain malfunction that required further surgery.
Asymptomatic Patients
Four patients with PF tumor were asymptomatic for hydrocephalus at presentation. These patients underwent MR imaging for neurofibromatosis type 1, whole-body fever work-up, possible Bell palsy, and chronic headaches. Three of these patients electively underwent surgical resection of the tumor and 1 underwent focal CyberKnife.
Sedation Status
Sedation status was matched for all patients and their respective age-matched controls. For all patients with symptomatic hydrocephalus who underwent follow-up ASL perfusion, the sedation status was also identical for both baseline and follow-up MR imaging.
Cerebral Blood Flow
Controls.
For the 16 age-matched and sedation-matched healthy controls, there was no correlation between age and CBF of the cerebrum at the vertex (P = .884). Similarly, there were no correlations between age and perfusion at more specific sites—that is, the putamen (P = .920), thalamus (P = .244), or periventricular white matter (P = .297).
Baseline CBF.
CBF for all interrogated cerebral regions is summarized in Table 2. Patients with symptomatic hydrocephalus had lower CBF than healthy controls for all brain regions interrogated (P < .001) (Fig 2). No perfusion difference was seen between asymptomatic patients with PF tumor and healthy controls (P = 1.000).
Cerebral blood flow in healthy controls (n = 16) and patients with PF Tumor (n = 23)
Comparison of ASL-CBF among controls, asymptomatic patients, and patients with hydrocephalus.
Baseline and Follow-Up Comparison
Of 17 patients who showed symptomatic relief after neurosurgical intervention, 16 had both baseline and follow-up ASL perfusion. The median time between intervention and follow-up MR imaging used in the comparison analysis was 27 days (range, 1 day to 7 months). The median CBF increased after alleviation of obstructive hydrocephalus (P < .002) (Table 3 and Fig 3). Examples are shown in Figs 4 and 5.
Cerebral blood flow before and after alleviation of obstructive hydrocephalus (n = 16)a
ASL-CBF values before and after alleviation of obstructive hydrocephalus.
ASL perfusion of a 6-year-old girl presenting with hydrocephalus from a diffuse intrinsic pontine glioma. A, The patient presented with acute symptoms, including headache, nausea/vomiting, and somnolence. Enlarged ventricles and periventricular edema were noted and low CBF of 9, 13, 8, and 6 mL/100 g/min at the cerebral vertex, putamina, thalami, and periventricular white matter, respectively. A shunt was placed a day after the MR imaging. B, Improved CBF (30, 40, 36, 27 mL/100 g/min) and resolution of acute symptoms were noted in the respective brain regions a month later. Note the shunt catheter in place (arrow) and residual ventricular enlargement and edema.
ASL perfusion of a 5-year-old boy presenting with acute hydrocephalus due to aqueduct obstruction from a tectal glioma. A, The patient presented with acute gait abnormality, nausea/vomiting, and somnolence. Enlarged ventricles and low global CBF of 16, 20, 17, and 8 mL/100 g/mL were seen at the cerebral vertex, putamina, thalami, and periventricular white matter, respectively. The patient underwent third ventriculostomy a day later and subsequently showed symptomatic relief. B, Two months later, improved CBF was seen, with CBF of 43, 58, 50, and 32 mL/100 g/min in the respective brain regions. Note a CSF jet at the patent third ventriculostomy site (arrow).
Two patients continued to demonstrate elevated ICP after tumor resection due to ventriculoperitoneal shunt complications (peritoneal contamination from gastrostomy) and extraventricular drain malfunction that required a new shunt placement or revision. Therefore, these patients were only included in the preintervention CBF analysis. For these 2 patients, CBF values were low during elevated ICP. For example, the patient with elevated ICP due to shunt complications from peritoneal contamination showed further decrease in perfusion with CBF values of 10, 15, 12, and 6 mL/100 g/min at the cerebral vertex, putamen, thalamus, and periventricular white matter, respectively. MR imaging performed several hours after shunt revision showed mild improvement with CBF of 21, 33, 33, and 13 mL/100 g/min in the respective brain regions. The other patient also showed low CBF during extraventricular drain malfunction with improvement after a new shunt placement (Fig 6).
Serial ASL perfusion of a 10-year-old boy with hydrocephalus from medulloblastoma. A, The patient presented acutely with headaches and nausea/vomiting. Relatively low CBF of 29, 50, 45, 20 mL/100 g/min, compared with controls, was noted in the cerebral vertex, putamina, thalami, and periventricular white matter. The patient underwent PF tumor resection and extraventricular drain placement 2 days later. B, One day after the surgery, the patient continued to have high ICP despite extraventricular drain placement and showed low CBF of 22, 34, 30, and 12 mL/100 g/min in the respective brain regions. C, The symptoms continued to worsen with high ICP and further decrease in CBF of 17, 31, 18, and 10 mL/100 g/min 2 days later. Due to extraventricular drain malfunction, a new ventriculoperitoneal shunt was subsequently placed. D, Several months later, the patient presented for routine surveillance of medulloblastoma with a functioning ventriculoperitoneal shunt and decreased ventricular size and was clinically asymptomatic at this time. While some perfusion alteration may be expected from interval chemotherapy and radiation, it has, nevertheless, improved, with a CBF of 36, 44, 34, 21 mL/100 g/min in the respective brain regions.
Ventricular Size and Other Parameters
The bifrontal ventricle size ranged from 35 to 65 mm in our patients with hydrocephalus at presentation. CBF at each site did not correlate with sex (P > .082) or tumor type (P > .203). Among the patients with tumor, the distance between the frontal horns inversely correlated with CBF of the cerebrum (P = .036), but not the putamen (P = .156), thalamus (P = .111), or periventricular white matter (P = .121).
Among patients with clinical improvement following relief of symptomatic hydrocephalus, ventricular size showed a small but statistically significant decrease, with a median change of 0.8 mm (range, 0.4–13.3 mm) (P = .002).
Discussion
Reduced ASL-CBF was seen in children presenting with acute hydrocephalus from PF tumors compared with controls, with restoration of CBF close to the normal range after alleviation of hydrocephalus. Prior studies have shown that when ICP increases, disturbance in autoregulatory vasomotor capacity can reduce cerebral perfusion pressure, where a pronounced decrease in CBF can result at cerebral perfusion pressure below 40–50 mm Hg.17,18 Because CBF was not significantly reduced in patients with PF tumors but without signs of raised ICP, CBF changes in our study were likely driven primarily by ICP effects rather than the presence of a mass lesion.
Infants and young children with hydrocephalus often present with nonspecific symptoms32; therefore, the distinction between ventriculomegaly and hydrocephalus with increased ICP is critically important. Given that many different etiologies for ventriculomegaly (underdevelopment, atrophy, metabolic diseases, and others) exist and ventricular size does not reliably identify symptomatic hydrocephalus,11⇓⇓–14,16 a method that reflects alterations in cerebral hydrohemodynamics is desired. To our knowledge, this is the first study to report the use of ASL perfusion as a marker for symptomatic hydrocephalus. In contrast to nuclear medicine, CT and MR imaging contrast perfusion, or 2D phase-contrast MRA methods, ASL perfusion does not require contrast or radiation, provides quantitative CBF measurements directly at the tissue level, and is technically easy to acquire at a relatively short scan time.
The CBF of our hydrocephalus cohort (34.3 ± 14.9 mL/100 g/min) is consistent with a prior report by Hayashi et al,33 which showed low hemispheric CBF (32 ± 6 mL/100 g/min) by using 133Xe CT in adults who presented with hydrocephalus after aneurysm rupture and correlative high ICP levels (36 ± 6 mm Hg). Leliefeld et al23 also reported low CBF (25 ± 11 mL/100 g/min) calculated from carotid and basilar artery flow rates by using 2D phase-contrast MRA in children with hydrocephalus. The lower CBF estimated in that study may be attributed to some combination of the younger age (range, 1 day to 7 months) of their cohort that might have had less robust autoregulatory mechanisms or more compressible parenchymal vessels within an immature brain, low signal due to a 2D phase-contrast MRA technique, and variable etiologies of hydrocephalus (intraventricular hemorrhage, arachnoid cyst, mucopolysaccharidosis, and various obstructive lesions). Of note, CBF of our healthy pediatric controls (62.3 ± 6 mL/min/100 g) is consistent with a normal mean CBF range of 53–71 mL/min/100 g in children 2–19 years of age measured by 133Xe SPECT34 but higher than 133Xe CT–based mean CBF of 50 mL/min/100 g reported for healthy adults.33
CBF was reduced for all brain regions in patients presenting with symptomatic hydrocephalus. Studies have shown that ventriculomegaly may directly decrease CBF via mechanical distortion and reduced vessel caliber.35,36 Alternatively, reduced brain compliance and, thereby, narrow-capacitance vessels, induced by ventricular enlargement, may decrease CBV and CBF, similar to a hydrodynamic mechanism proposed by Greitz.37,38 However, it is noteworthy that ventricular size inversely correlated with CBF of the cerebrum at the vertex, but not in the other brain regions. It is possible that deep gray and periventricular regions are more vulnerable to mechanical effects with pronounced CBF changes, even with mild ventriculomegaly. This finding unlikely reflects underlying regional pressure differences because no evidence for transmantle pressure gradient has been shown in hydrocephalus due to viscoelasticity of the brain substance.8,39
Concepts of hydrocephalus are complex and continue to evolve.8,9,37,38 They include the following: the conventional major CSF pathway, including the original work of Dandy and Blackfan40 on bulk flow theory and distinction of obstructive and communicating hydrocephalus; and a minor CSF pathway that describes interactions of brain compliance, CSF and arteriole pulsations, vascular capacitance/pulse pressures, and capillary absorption.8,9,37,40⇓–42 Given the complex pathophysiology, understanding the physiology of hydrocephalus remains a challenging neurosurgical problem.
While ventricular size remains important for the work-up of hydrocephalus, prior studies have shown that it may not reliably identify abnormal ICP11⇓–13,15 or resorptive capacity for CSF,11 particularly in cases of chronic hydrocephalus or shunt failure in which brain compliance may be altered from injury, periventricular gliosis, and other reactive changes.12 Our results suggest that ASL perfusion identifies changes in underlying tissue physiology and may, therefore, be a useful adjunct to conventional MR imaging without the need for contrast or radiation exposure.
There are a few limitations of this study. Direct CSF pressure recordings that correspond to CBF changes would be desirable; however, pressure measurements are not routinely obtained during resection of PF tumors or at the time of MR imaging and, therefore, were not feasible. While ASL-CBF was altered in the uncompensated state and was restored after CSF symptomatic relief in this population of pediatric patients with PF tumors, baseline perfusion metrics and deficit patterns may vary to some degree in other models of hydrocephalus, such as normal-pressure hydrocephalus of older adults; chronic, compensated, or nonprogressive hydrocephalus; and various conditions of hydrocephalus associated with prior hemorrhage/injury, brain malformations, and metabolic disorders and congenital chromosomal disorders. However, CBF changes across time intervals, nevertheless, could help identify altered physiologic conditions that relate to ICP status and other challenging cases of ventriculomegaly (On-line Figs 1–3). Finally, general effects of sedation on childhood cerebral perfusion and metabolism are relatively unknown27; however, this lack of knowledge is unlikely to have impacted our study, given that the sedation status was matched for baseline and follow-up ASL scans, and for the patient and control groups.
Conclusions
ASL-CBF was reduced in patients with symptomatic hydrocephalus and restored after surgically mediated alleviation. ASL perfusion MR imaging may serve a future role in the evaluation of hydrocephalus, as a potential noninvasive method to follow changes in ICP.
Footnotes
Paper was previously presented in part in abstract form at: Meeting of American Association of Neurological Surgeons, April 14–18, 2012, Miami, Florida; and Annual Meeting of the Society for Pediatric Radiology, April 16–17, 2012; San Francisco, California.
References
- Received October 22, 2013.
- Accepted after revision November 13, 2013.
- © 2014 by American Journal of Neuroradiology