Abstract
SUMMARY: IgG4-related disease is characterized by histologic fibrosis with IgG4-positive plasma cell infiltration. Our study evaluated MR imaging features of IgG4-related disease in the head and neck and brain. Images from 15 patients were retrospectively evaluated for the location, signal intensity, and enhancement patterns of lesions. Lacrimal gland enlargement was observed in 8 cases. Other lesions included orbital pseudotumor in 5, pituitary enlargement in 5, and cranial nerve enlargement in 7; the infraorbital nerve was involved in 4. All lesions were hypointense on T2-weighted images, which is typical for IgG4-related lesions. Multiple sites were involved in the head and neck and brain in 11 patients. The diagnosis of IgG4-related disease should be considered in a patient presenting with T2 hypointense lacrimal gland, pituitary, or cranial nerve enlargement, or a T2 hypointense orbital mass, especially if multiple sites in the head and neck are involved in the presence of elevated serum IgG4.
ABBREVIATIONS:
- AIP
- autoimmune pancreatitis
- HN
- head and neck
- IgG4
- immunoglobulin G4
- IgG4-RD
- IgG4-related disease
- IPT
- inflammatory pseudotumor
- MD
- Mikulicz disease
- SS
- Sjögren syndrome
- V1
- first division of the trigeminal nerve (CN V)
- V2
- second division of the trigeminal nerve
IgG4 is a subclass of IgG that accounts for less than 6% of total serum IgG. IgG4-RD is an autoimmune condition associated with increased serum IgG4 levels and mass lesions with characteristic histologic features.1⇓⇓⇓⇓⇓⇓⇓–9 It typically progresses over long periods of time in single or multiple organs and is usually responsive to steroid therapy. In 2001 and 2002, Hamano et al reported AIP associated with increased serum IgG4 levels1 and characterized histologically by lesions with abundant IgG4-positive plasma cell infiltration, fibrosis, and obliterative phlebitis.2 Subsequently, many extrapancreatic lesions, including sclerosing cholangitis, sclerosing sialadenitis, retroperitoneal fibrosis, interstitial pneumonia, and mediastinal fibrosis, have been reported to show similar histopathologic features and association with increased serum IgG4 levels2⇓⇓⇓–6; such lesions may develop with or without AIP. In 2005, Yamamoto et al reported the presence of IgG4-positive plasma cells in lesions of MD and proposed MD as another type of IgG4-related disease.7,8
In 2006, clinical diagnostic criteria for AIP were proposed,10 including 1) diffuse or segmental narrowing of the main pancreatic duct, with irregular walls and diffuse or localized enlargement of the pancreas on imaging; 2) high-serum F-globulin, IgG, or IgG4 concentration or the presence of autoantibodies, such as antinuclear antibodies and rheumatoid factor; and 3) marked interlobular fibrosis and prominent infiltration of lymphocytes and plasma cells to the periductal area, occasionally accompanied by lymphoid follicles in the pancreas. For diagnosis, criterion 1 must be present together with criterion 2 and/or 3. It is also necessary to exclude malignant diseases such as pancreatic and biliary cancers.
In 2008, the Japanese Society for Sjögren's Syndrome proposed diagnostic criteria for IgG4-related MD (On-line Table 1),11 and in 2010, a research group of the Japanese Ministry of Health, Labor, and Welfare established the diagnostic criteria for IgG4-RD. The 2 main criteria are 1) serum IgG4 concentration >135 mg/dL, and 2) >40% of IgG+ plasma cells being IgG4+ and >10 cells/high-powered field of biopsy sample12 in any affected organ.
Regarding HN lesions complicating AIP, the incidence of lacrimal gland and salivary gland enlargement have been reported as 12.5% and 25.9%, respectively.13 HN lesions of IgG4-RD without AIP have also been reported, however, to date, only a few studies have reported imaging findings of IgG4-RD in the HN and brain,14⇓⇓–17 most from Japan. This study evaluated MR imaging features of IgG4-RD in the HN and brain, using data collected from multiple institutions.
Case Series
The institutional ethics review board approved the study and waived informed consent. MR images of 15 patients with pathologically proved IgG4-RD in HN and brain (age, 35–73 years; mean, 60.5 years; 12 men and 3 women), acquired from April 2006 to March 2011, were collected from 11 institutions.
The duration of multiphasic (n = 14) or multisystemic (n = 13) symptoms in each patient ranged from 6 months to 20 years. In 1 patient with orbital involvement, the symptom was uniphasic. Symptoms associated with the HN or brain lesions in the 15 patients were exophthalmos (n = 10), visual acuity loss (n = 2), headache (n = 2), mild dysesthesia of the cheek (n = 2), vertigo (n = 1), dizziness (n = 1), diabetes insipidus (n = 1), and sicca syndrome (n = 1). All 15 patients had high serum IgG4 levels (>135 mg/dL) and histopathologic confirmation of IgG4-RD; histologic specimens were obtained from the lacrimal gland (n = 8), eyelid (n = 1), both the middle ear and the nasal cavity (n = 1), and chest or abdominal organs (n = 5).
Two experienced neuroradiologists (K.T. and H.O.) evaluated brain and head and neck MR images with consensus for the location, extent, and T1- and T2-signal intensity characteristics of lesions. Enhancement patterns of the lesions were evaluated in 12 of 15 patients who received gadolinium contrast-enhanced study. Additional bony changes around the lesions were also evaluated in 2 patients for whom CT images were available.
Imaging parameters were variable among the 15 patients, as MR images were collected from 11 different institutions. All MR studies were obtained using 1.5-T MR systems (GE Healthcare, Siemens, Philips, or Toshiba), and routine T1-weighted imaging, T2-weighted imaging, and, in some cases, T1-weighted imaging with contrast enhancement were examined in each institution. T1-weighted images were acquired with the following parameters: TR/TE = 580–400/10–15 ms; section thickness, 3–4 mm; FOV, 16–18 cm; matrix, 256 × 224, 288 × 192, 288 × 256, or 256 × 195. T2-weighted images were acquired with the following parameters: TR/TE = 4000–3800/107–100 ms; section thickness, 3–4 mm; FOV, 16–18 cm; matrix, 256 × 256, 288 × 192, 288 × 256, or 352 × 275; echo-train length, 10–19. In 4 patients, fat suppression technique was added in T2-weighted imaging. Fat suppression technique was used in 6 of 12 patients who had enhanced study.
The clinical records and findings on CT of the chest and abdomen were also evaluated in each institution to check for IgG4-RD lesions in other areas.
Results
A summary of affected sites, including other organs, is shown in On-line Table 2. Involved sites in the HN and brain included the lacrimal glands (n = 8), other areas of the orbit (n = 5), cranial nerves (n = 7), pituitary gland (n = 5), dura mater (n = 4), and submandibular glands (n = 3). In 11 of the 15 patients, multiple sites were involved in the HN and brain. The associations of orbital involvement (lacrimal gland enlargement and/or intraorbital masses) and cranial nerve enlargement, and of enlargement of both cranial nerve and pituitary gland, were most common (4 cases each). All of the HN and brain lesions were hypointense to brain parenchyma on T2-weighted images. Six of the 15 patients also had AIP. The other sites of systemic involvement included the lung (n = 3), hilar lymph nodes (n = 2), mediastinum (n = 1), bile ducts (n = 1), kidneys (n = 2), and retroperitoneum (n = 4).
Eight patients showed bilateral enlarged lacrimal glands with a homogeneous signal on T1- and T2-weighted images (Fig 1 A). These lesions showed homogeneous enhancement on gadolinium contrast-enhanced study. No apparent signal change or destruction of the adjacent bone was present; however, mild pressure erosion of the lacrimal gland fossa was identified on CT in 1 patient.
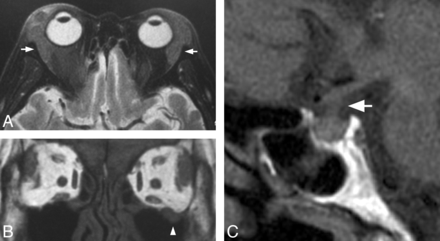
A 73-year-old man with lacrimal gland, infraorbital nerve, and pituitary lesions. A, Axial T2-weighted image shows diffuse swelling of bilateral lacrimal glands showing hypointensity of the lesion (arrow). B, Coronal T1-weighted image shows thickening of the left infraorbital nerve (arrowhead). C, Sagittal T1-weighted image shows enlargement of the pituitary stalk (arrow) and disappearance of hyperintensity of the posterior lobe.
Five patients showed bilateral intraorbital masses that were distinct from enlarged lacrimal glands and had well-defined borders (Figs 2 A and 3 A). These masses were extraconal; they appeared as multiple nodules in 2 patients, a single mass in each orbit in 2 patients, and diffuse symmetrical infiltration of the peripheral orbital fat outside the muscle cone in 1 patient (Fig 3). Two of the 5 patients also had both extra- and intraconal masses.
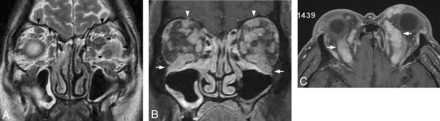
A 67-year-old man with intraorbital, infraorbital nerve, and frontal nerve lesions. A, Coronal T2-weighted image shows multiple hypointense nodules in the bilateral orbits, both inside and outside the muscle cone (arrowheads). Bilateral infraorbital and frontal (arrowheads) nerves show concentric thickening. B, Coronal gadolinium-enhanced fat-suppressed T1-weighted image shows marked enhancement of the lesions. Enhancement and concentric thickening of bilateral infraorbital (arrows) and frontal (arrowheads) nerves are noted. C, Axial gadolinium-enhanced fat-suppressed T1-weighted image shows diffuse concentric thickening of bilateral frontal nerves (arrows).
A 66-year-old man with intraorbital and dural lesions. A, Coronal gadolinium-enhanced fat-suppressed T1-weighted image shows diffuse lesions outside the muscle cone in the bilateral orbits (arrows). B, Axial T2-weighted image shows marked T2-shortening of the extraconal lesions (arrows) and diffuse thickening of the dura mater (arrowheads). C, Gadolinium-enhanced fat-suppressed T1-weighted image shows distinct enhancement effect of the thickened dura mater (arrowheads).
Seven patients showed cranial nerve involvement. The infraorbital nerve, a branch of the V2, was involved in 4 cases (Figs 1B and 2A, -B) with diffuse concentric thickening of the nerve. In 1 case in which CT images were available, widening of the infraorbital bony canal was noted. Involvement of the frontal nerve, a branch of the V1, was observed in 1 case (Fig 2); the lesion appeared as a tube-like mass at the upper aspect of the superior orbital levator muscle, from the orbital apex to the inner angle of the eyelid (Fig 2C). Mass lesions of the cavernous sinus, probably caused by thickening of the V1 or V2, were noted in 3 cases. In 1 patient, the tympanic and mastoid segments of the facial nerve was thickened with pathologic proof, as >40% of IgG+ plasma cells being IgG4+ infiltration in the middle ear.
Five patients showed enlargement of the pituitary stalk and posterior lobe of the pituitary gland (Fig 1C). These lesions were low-intensity on T2-weighted imaging and showed contrast enhancement. The anterior lobe was also enlarged in 2 of the 5 patients. Hyperintensity of the posterior lobe was absent on T1-weighted images in all 5 cases (Fig 1C). Four patients showed thickening of the dura mater, which was diffuse (n = 2) or localized (n = 2). The thickened dura matter showed marked hypointensity on T2-weighted imaging and contrast enhancement (Fig 3B, - C).
In 1 case, concentric thickening of the vertebral artery was observed, along with a facial nerve lesion, paranasal sinusitis, otitis media, and a nerve root lesion. The flow void of the vertebral artery was intact without any stenosis.
Bilateral diffuse swelling of the submandibular glands was physically detected in 3 cases; bilateral enlargement was detected in 1 of these cases on MR imaging.
Six patients had AIP lesions and 6 patients had other abdominal IgG4-related involvement, including retroperitoneal fibrosis in 4, renal involvement in 2, and bile duct involvement in 1. Six patients had chest lesions: pulmonary lesions in 4, hilar lymphadenopathy in 2, and mediastinal fibrosis in 1. Five patients had coexistent lesions of the chest and abdomen.
Discussion
In the head and neck and brain, manifestations of IgG4-RD include enlargement of salivary glands and lacrimal glands, IPT, pituitary lesions, thickening of the dura mater/pachymeningitis, and thyroid lesions.15 We observed enlargement of the lacrimal gland in 8 cases of MD; according to the 2010 criteria for IgG4-RD, we now recognize that these patients probably had IgG4-related disease of the HN.18
MD was previously thought to be a subtype of SS, but some clinical differences between MD and SS have been defined,19 notably that SS-A and SS-B antibodies and the sicca symptom are absent in MD. After the establishment of the concept of IgG4-RD, MD is considered as separate from SS and part of the IgG4 disease spectrum.
IPT can be an intraorbital manifestation of IgG4-RD and may cause intraconal, conal, or extraconal masses. Most cases of IPT, however, are not part of the IgG4-RD spectrum. Thickening of branches of the trigeminal nerve was also a common periorbital finding, with preferential involvement of the infraorbital nerve noted in our cases. Prior studies have described IgG4-related inflammatory pseudotumor of the trigeminal nerve as a homogeneously enhancing soft-tissue mass involving the skull base along the second and third divisions of the trigeminal nerves, extending to the masticator space via the foramen ovale.14
Pathologic studies of pituitary lesions associated with IgG4-RD have reported fibrosclerosis with infiltration of lymphocytes and plasma cells.20 In these cases, “infundibulohypophysitis” of the anterior and posterior lobes of the pituitary gland was observed, leading to enlargement of the pituitary stalk and gland. Thus, lymphocytic hypophysitis may be due to IgG4-RD in some cases, and appropriate laboratory tests for IgG4 should be carried out for this condition.
Dural thickening with marked T2-shortening was noted in our cases, presumably related to fibrosis or sclerosis. In a reported case, localized dural thickening was noted, with hypointensity on T2-weighted images, pathologically proved to be a IgG4-related manifestation, and diagnosed as IgG4-related sclerosing pachymeningitis.21 At least some of the cases described in the literature as idiopathic hypertrophic pachymeningitis probably belong to this disease category.
In our study, we observed concentric thickening around the vertebral artery in 1 case. Because lesions have been found around the arteries in the abdomen and chest in IgG4-RD, it is not surprising that lesions would also form around the intracranial or pericranial arteries, such as the vertebral artery. One study reported dolichoectasia of the vertebrobasilar arteries, caused by IgG4-RD.22
When lesions involving the HN and brain with T2 shortening are present, particularly in the anatomic locations described herein, then investigations for elevated IgG4 in serum, systemic manifestations, and consistent pathologic findings should be sought for more specific diagnosis.
There are some limitations to our study. First, our case series was not consecutive, was collected from multiple institutions, and does not allow the overall disease incidence or the incidence of each HN and brain site of involvement to be inferred from the results. Second, as imaging parameters were not consistent among the different MR machines used, quantitative evaluation of signal intensity was not possible, and it was not feasible to detect any differences that may have occurred in these parameters according to disease duration.
Conclusions
IgG4-RD is a multisystemic disease that can involve many organs, including the orbit, nerves, pituitary gland, dura, and meninges. This diagnosis should be considered in the setting of T2-hypointense enhancing lesions of the lacrimal glands and branches of the trigeminal nerves in particular. It is important to increase awareness of IgG4-RD among neuroradiologists, neurosurgeons, and neurologists, as well as otorhinolaryngologists and ophthalmologists, so that appropriate diagnostic evaluation can be performed. When lesions involving the HN and brain with T2 shortening are present, investigation of the IgG4 serum level and imaging of the chest and abdomen are recommended.
Acknowledgments
We thank Toru Takeshita, MD, Osaka City University; Yoshiyuki Watanabe, MD, Osaka University; Hiroshi Nobusawa, MD, Kawasaki Saiwai Hospital; Satoshi Matsushima, MD, Jikei University; Satoshi Tatsuno, MD, Tokyo Dental College Ichikawa General Hospital; and Fumiaki Ueda, MD, Kanazawa University for assisting with the enrollment of patients.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received November 30, 2011.
- Accepted after revision March 16, 2012.
- © 2012 by American Journal of Neuroradiology