Abstract
BACKGROUND AND PURPOSE: One former study reported higher prefrontal N-acetylaspartate (NAA) levels in patients with Asperger syndrome (AS). The objective of the current study was to test the hypothesis that patients with AS would have higher dorsolateral prefrontal and anterior cingulate cortex NAA/creatine (Cr) and that NAA/Cr would be correlated with symptom severity.
MATERIALS AND METHODS: NAA/choline (Cho), NAA/Cr, and Cho/Cr values revealed by 1H-MR spectroscopy in 14 right-handed male patients with AS (6 medicated with risperidone), 17–38 years of age, diagnosed by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria were compared with those of 21 right-handed male controls frequency-matched by age and intelligence quotient scores.
RESULTS: Patients with AS had significantly higher anterior cingulate NAA/Cho levels (z = −2.18, P = .028); there was a statistical trend for higher anterior cingulate NAA/Cr (z = −1.81, P = .069) that was significant when only the unmedicated patients with AS were taken into account (z = −1.95, P = .050). There were no significant differences in dorsolateral prefrontal MR spectroscopy values.
CONCLUSIONS: Our findings show that individuals with AS had higher NAA/Cho levels in the right anterior cingulate compared with healthy controls and that higher anterior cingulate NAA/Cho levels were correlated with higher Yale-Brown Obsessive Compulsive Scale total scores.
A sperger syndrome (AS) is a pervasive developmental disorder characterized by similar qualitative abnormalities in reciprocal social interaction that typify autism. As in autism, individuals with AS have intense problems in restricted, stereotyped, and repetitive patterns of behaviors, interests, and activities.1 Although phenomenologically different from obsessive-compulsive disorders, the obsessional/repetitive behaviors in AS are amenable to measures of obsessive symptoms used in previous neuroimaging studies of AS.
MR spectroscopy has many advantages in developmental psychiatry: lack of ionizing radiation and known side effects,2 provision of in vivo quantitative biochemical information, high spatial resolution, and opportunity for integration of understanding of brain structure with brain metabolism.3 To date, there has been only been 1 previously published MR spectroscopy study involving patients with AS.4 In the study by Murphy et al,4 1H-MR spectroscopy was used to compare the prefrontal and parietal N-acetylaspartate (NAA), choline (Cho), creatine (Cr), and phosphocreatine levels in 14 patients with AS with those of 18 healthy controls. The results indicated that the prefrontal NAA, Cho, Cr, and phosphocreatine levels of patients with AS were significantly higher than those in controls, and prefrontal NAA and prefrontal Cr were reported to be positively correlated with symptom severity.4
Both 31P and 1H have been used to evaluate the metabolite levels in various brain regions of patients with autism. Results of these studies have not been conclusive, reporting differences in various regions of the brain. The first MR spectroscopy study of patients with autism found decreased levels of phosphocreatine in the dorsolateral prefrontal cortex.5 The authors postulated that a hypermetabolic energy state and undersynthesis of brain membranes may underlie this in individuals with autism. Some studies found decreased NAA levels in the hippocampus-amygdala,6 temporal cortex,7 and cerebellum8 of children with autism. Another study reported an association between 1H-MR spectroscopy Cho/Cr ratios and severity of autism.9 The authors concluded that this might be due to increased cellular proliferation. On the other hand, a further study reported no differences in the NAA/Cho ratio.10
To date, neuroimaging data indicate that the anterior cingulate and dorsolateral prefrontal cortex may represent brain regions critical for our understanding of the neurobiology of autism, as well as of AS.11 A number of studies suggested that anterior cingulate cortex might be part of the “emotional brain,” and this might also be related to the theory of mind deficits in individuals with autism.12 Morphometric and positron-emission tomography (PET) studies have also pointed to smaller anterior cingulate volumes and reduced metabolism in subjects with autism.13 Lesion studies indicate social cognition and theory of mind deficits in patients with ventromedial prefrontal cortex lesions14; likewise, functional MR imaging studies have reported atypical frontal activation in theory of mind tests.15
In the present study, our hypothesis was that patients with AS, diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria, had higher dorsolateral prefrontal and anterior cingulate cortex NAA/Cr levels and that NAA/Cr would be correlated with symptom severity. To test this hypothesis, we evaluated the levels of brain metabolites in these brain regions in patients with DSM-IV-based AS and compared these levels with those in healthy controls; finally, we evaluated the correlations of these neurometabolites with a measure of obsessional repetitive behaviors.
Materials and Methods
Subjects
All subjects were evaluated by a team of 2 psychiatrists and a psychologist and met DSM-IV criteria for AS. Parents of all subjects were interviewed to establish the developmental history and symptom onset and cessation. None of the subjects had language delays (defined as single words by age 2 and communicative phrases by age 3). The diagnosis of AS met the consensus of all 3 clinicians based on separate clinical evaluations. None of the subjects were excluded due to lack of consensus. All the subjects were screened by using the Structural Clinical Interview for DSM-IV Axis I diagnosis (SCID-I) for any other DSM-IV diagnoses, all with negative results. Subjects were recruited from the consecutive outpatient psychiatry rosters of the 2 university psychiatry departments at Ankara University School of Medicine and Hacettepe University School of Medicine, Ankara, Turkey. All subjects had intelligence quotient (IQ) scores higher than 70 on the Weschler Adult Intelligence Scale, Revised (WAIS-R), based on application of the entire WAIS-R battery. Inclusion criteria for patients with AS were presence of DSM-IV AS diagnosis and being male and right-handed; subjects were 17–38 years of age. Exclusion criteria for AS subjects included presence of comorbid psychiatric and neurologic conditions.
Controls were likewise screened for DSM-IV diagnoses by SCID-I. Inclusion criteria for control subjects were being male and right-handed. Exclusion criteria for controls were presence of psychiatric and neurologic conditions.
Before the commencement of the study, a complete description of it was given to the prospective subject, both patients with AS and controls. After a complete description of the study was given to the subjects, written informed consent was obtained. Ankara University School of Medicine ethics committee, with a federally sponsored United States Department of Health and Human Services Office of Human Subjects Research Protections assurance, approved the study.
Measures and Diagnoses
Structural Clinical Interview for DSM-IV Diagnosis (SCID).
SCID is a semistructured interview for DSM-IV diagnoses, completed by trained interviewers. The Turkish version of the SCID has been translated by Özkürkçügil et al,16 with good reliability. It consists of 6 modules and usually takes 25–50 minutes to complete.
Yale-Brown Obsessive Compulsive Scale.
The Yale-Brown Obsessive Compulsive Scale (Y-BOCS) interview developed by Goodman et al17 was used to evaluate the severity of obsessive and compulsive symptoms. Y-BOCS consists of 19 items, but the first 10 items are used to calculate the total score. Y-BOCS is a semistructured interview, and the questions are asked according to the directions. The clinician-interview version of Y-BOCS questions were asked by the examiner.
We decided to use Y-BOCS to evaluate obsessions and repetitive behaviors in patients with AS for 2 reasons. First, although core symptoms in autism-spectrum disorders include obsessional/repetitive behaviors and although such behaviors may be reduced with serotonin-reuptake inhibitors, it has also been argued that obsessional/repetitive behaviors seen in autism-spectrum disorders and obsessive-compulsive disorder may be phenomenologically different.18, 19 Nevertheless, in this study, we aimed to investigate the relationship of obsessive behavior scores and brain metabolism in AS. Second, because the only former MR spectroscopy study that investigated these phenomena in subjects with AS used Y-BOCS, we chose to use the same instrument and aimed to investigate whether we could replicate these former findings.4
MR Spectroscopy Imaging
All scanning was performed with a 1.5T scanner (Magnetom Symphony, Syngo Version VA21C; Siemens Medical Systems, Erlangen, Germany) equipped with high-performance gradients (maximal gradient strength, 40 mTm−1 and maximal slew rate, 200 mTm−1/ms) using a standard head coil. Before performing 1H-MR spectroscopy, we acquired gradient-refocused echo T1-weighted images in coronal, axial, and sagittal planes of the whole brain by using a gradient-echo pulse sequence (section thickness, 3 mm; FOV, 23 cm; TR, 585 ms; TE, 12 ms; flip angle, 70°; matrix, 101 × 192) to get an anatomic image to select a volume of interest (VOI) to perform localized 1H-MR spectroscopy. Point-resolved selective spectroscopy 2D chemical shift imaging (2D-CSI) was performed to measure the metabolite ratios in the right prefrontal cortex, anterior cingulate cortex, and amygdala, with the scanning parameters of TR, 1500 ms; TE, 270 ms; and a VOI, 1 × 1 × 1 cm3. We tried to minimize sinus-induced MR susceptibility artifacts in the temporal lobe concerning the amygdala region by positioning the subject in a hyperextended position. The CSI sequence produced a 16 × 16 transversely oriented matrix that was defined by phase-encoding with an FOV of varying dimensions to allow optimal measurement in the areas of concern. The field inhomogeneity achieved in automated nonlocalized multiple-angle-protection shimming resulted in water peak line widths of less than 8 Hz in the VOI. The 2D-CSI raw data were filtered with the Hanning and Gaussian filters on the spatial domain and chemical shift domain, respectively before the Fourier transformation. A frequency domain curve fitting was subsequently used for quantification with the assumption of Gaussian line shapes, by using the standard Syngo Spectroscopic Evaluation software package (Siemens) provided with the MR imaging system. We used areas under the curve to compute NAA, Cr, and Cho values.
The VOIs were located in the right anterior cingulate cortex and right dorsolateral prefrontal cortex. The voxels in the anterior cingulate cortex corresponded to Brodmann area (BA) 24 and 32, and in the dorsolateral prefrontal cortex, to BA 9 and 46, based on the Talairach atlas.20 We selected right-sided voxels of interest for 2 reasons. First, previous studies have suggested that subjects with autism-spectrum disorders might have decreased activity in the right cortical regions.12 Second, Murphy et al4 investigated right frontal and parietal regions, and we investigated whether we could replicate their findings. All patients with AS and control subjects cooperated fully during the procedure.
Data Analysis
To compare the NAA/Cr, NAA/Cho, and Cho/Cr ratios of metabolites between groups, we used the nonparametric Mann-Whitney U test. To compute correlations of symptom severity and MR spectroscopy variables, we used the nonparametric Spearman rank correlation test. We also compared unmedicated subjects with controls separately. Two-tailed significance tests (P <. 05) are reported throughout.
Results
Fourteen patients with AS, 17–38 years of age (mean, 24.3 ± 7.1 years), were included in the study. All subjects were right-handed and male. Although 8 subjects were unmedicated and 6 subjects were on chronic risperidone treatment, there were no demographic differences between medicated and unmedicated subjects. Twenty-one right-handed male controls, 19–38 years of age (25.0 ± 5.0 years), were included in the study. WAIS-R full-scale IQ scores were similar in patients with AS (87.2 ± 10.9) and controls (90.1 ± 11.7). There were no significant age and WAIS-R full-scale IQ differences between the groups.
Patients with AS had significantly higher anterior cingulate NAA/Cho levels (z = −2.18, P = .028). There was also a statistical trend for higher anterior cingulate NAA/Cr (z = −1.81, P = .069), which was significant when only the unmedicated patients with AS were taken into account (z = −1.95, P = .050) (Table). There were no significant differences on dorsolateral prefrontal MR spectroscopy values.
Mean and SD, z scores (Mann-Whitney U test), and P values of age, WAIS-R full-scale IQ, and MR spectroscopy variables of patients with AS and controls
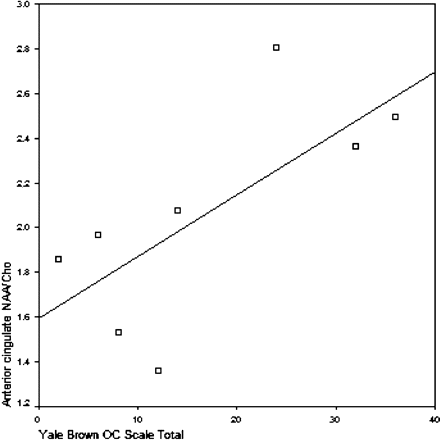
In patients with AS, Y-BOCS total score (higher scores reflecting the presence of pathology) was significantly correlated with anterior cingulate NAA/Cho values (r = 0 0.71, P = .047) and significantly negatively correlated with dorsolateral prefrontal NAA/Cho ratios (r = −0. 81, P = .015), only in the unmedicated subjects (Fig 1).
Relationship of anterior cingulate NAA/Cho and Y-BOCS total score in unmedicated patients with AS (n = 8) (Spearman ρ = 0.71, P = .047).
Discussion
Our findings indicate that patients with AS had significantly higher anterior cingulate cortex NAA/Cho. There was also a trend for higher anterior cingulate NAA/Cr, which was more significant in the unmedicated subjects; there were no differences in Cho/Cr. Our study hypothesis, that patients with AS would have higher dorsolateral prefrontal and anterior cingulate cortex NAA/Cr, was partially supported by the results obtained.
Our findings are, therefore, partially consistent with the 1 previous MR spectroscopy study involving subjects with AS that also reported higher NAA levels in the prefrontal cortex.4 However, not all previous MR spectroscopy studies involving subjects with autism are in agreement with our findings because some reported higher NAA levels5, 6 and others reported lower10 or unchanged levels.11 The differences in these findings may be attributable to methodologic differences, as in MR spectroscopy techniques and/or age and intelligence of the subjects involved. Some studies included subjects younger than 21 years of age,7, 8, 10 1 study involved subjects with mental retardation,11 and intelligence levels of the subjects were not described in 3 of the studies.7, 8, 11 In any case, whereas 31P measures the levels of the phospholipid precursors (phosphomonoesters) and the phospholipid breakdown products (phosphodiesters), as well as high-energy phosphate metabolism, 1H-MR spectroscopy measures the levels NAA and Cho.3 Thus, these 2 MR spectroscopy techniques do not necessarily measure the same variables, making studies conducted by using differing techniques difficult to compare.
Postmortem studies have found increased neuronal attenuation in the anterior cingulate cortex21 in subjects with autism. Another neuropathologic study had indicated abnormalities in the mini columnar organization of neurons in patients with AS and autism subjects, which could possibly lead to overconnected and insufficiently inhibited neural networks.22 Areas of aberrant white matter, evaluated by diffusion tensor imaging, were reported between regions activated in the theory of mind tests, including the anterior cingulate and amygdala.23 On the other hand, another study reported smaller anterior cingulate volumes related to lower metabolic activity.13 However, these authors argued that the smaller volume did not necessarily contradict increased neuronal attenuation because the reduced dendritic field could be associated with more densely packed cells. Our finding that unmedicated patients with AS might have higher anterior cingulate NAA supports these results.
High NAA levels might be related to increased neuronal attenuation or metabolic abnormality, glial hypoplasia, and/or abnormal synaptic pruning.24 NAA synthesis occurs in the mitochondria and is coupled to mitochondrial activity in adenosine triphosphate production.25 High NAA levels might be an indication of aberrant neural connections, suggested by the previously mentioned findings of neuropathologic and neuroimaging studies.
We found that NAA/Cho was significantly correlated with Y-BOCS total score in the unmedicated subjects. This was consistent with the former studies,4 which also found significantly higher Y-BOCS scores with prefrontal NAA concentrations. These authors suggested that obsessional behavior in patients with obsessive-compulsive disorder and AS might have a different neurobiologic basis. Nevertheless, we also found significant inverse correlation of Y-BOCS total scores with dorsolateral prefrontal NAA/Cho. Functional neuroimaging studies showed that whereas anterior cingulate functional activity increases in obsessive-compulsive disorders, dorsolateral prefrontal activity decreases.26 Baxter's obsessive-compulsive disorder model suggested that overactivity of ventromedial prefrontal cortex and underactivity of dorsolateral prefrontal cortex lead to repetitive behaviors.26 However, dorsolateral NAA/Cho was not significantly different between patients with AS and controls. The possible relations of various prefrontal cortical regions with different symptom dimensions need to be evaluated thoroughly in future studies.
The major limitation of the present study was the small sample size, which might increase the risk of both type 1 and type 2 errors, in rejecting the null hypothesis when it is true and failing to observe a difference when there is one, respectively. We used a nonparametric Mann-Whitney test with the assumption that normal distribution could not be achieved; the lack of difference in dorsolateral prefrontal cortex MR spectroscopy variables might also be related to the small sample size. The sample size was further reduced after we analyzed unmedicated subjects separately. These findings, therefore, need to be further evaluated by using larger sample sizes. One of the interesting findings of this study was that the differences between patients with AS and controls were more prominent in unmedicated subjects. Previous studies conducted with patients with schizophrenia suggested that antipsychotic treatment, particularly with atypical antipsychotic agents, might prevent the decrease of NAA levels during the course of the disorder, suggesting that these drugs might have neuroprotective effects.27, 28 However, in the present study, we did not have an a priori hypothesis concerning the drug effects, nor did we directly compare the subjects on or off medication due to small sample size. Therefore, this was a clear limitation of the study, and the possible effects of drugs on brain neurochemistry must be evaluated in future follow-up studies. The dorsolateral prefrontal VOI was in proximity to the skull; however, there was little interference in the spectra obtained from this region. Finally, our sample consisted of only male subjects; though AS is strongly prevalent in males,1 our results cannot be generalized to all patients with AS.
Conclusions
Our findings showed that individuals with AS had higher NAA/Cho in the right anterior cingulate compared with healthy control subjects and that higher anterior cingulate NAA/Cho levels were correlated with higher Y-BOCS total scores.
Footnotes
This work was supported by the Fogarty International Center/National Institute of Mental Health, Mental Health and Developmental Disabilities Research Training Program (D43TW05807) at the Children's Hospital Boston.
References
- Received January 5, 2007.
- Accepted after revision February 13, 2007.
- Copyright © American Society of Neuroradiology