Abstract
Summary: We report 2 cases of intraspinal germinoma associated with Klinefelter syndrome. In one patient, spinal cord atrophy was observed at the upper and lower ends of the intraspinal tumor. Brain atrophy was observed in both cases. Germinoma should be included in the differential diagnosis if an intraspinal tumor is observed in a patient with Klinefelter syndrome.
Klinefelter syndrome is a variation of sex chromosome disorder characterized by hypogonadism, gynecomastia, and azoospermia, and the most frequent karyotype is XXY.1 A patient with Klinefelter syndrome has an increased risk of developing malignant tumors such as a breast cancer, leukemia, and extragonadal germinoma.2,3 Most of the extragonadal germinomas, including those associated with Klinefelter syndrome, are located within the mediastinum.4,5 Germinomas in the central nervous system (CNS) associated with Klinefelter syndrome, especially in the spinal cord, are extremely rare disorders.2,6 We report 2 cases of intraspinal germinoma associated with Klinefelter syndrome. In addition, we reviewed the literature with reference to the radiologic features of intraspinal germinoma and clinical features of germinoma in the CNS associated with Klinefelter syndrome.
Case Presentation
Case 1
Patient 1 was a 35-year-old Japanese man. In September 1998 (when he was 30 years old), he suffered from weakness of the right lower extremity. In April 1999, he became aware of weakness of the left lower extremity and difficulty in urination. On MR images of the thoracic spine at an outside hospital, T2-weighted images (T2WI) revealed an area of high signal intensity in the spinal cord at the level of the seventh thoracic (T7) vertebral body (Fig 1A). Enlargement of the spinal cord was not observed. Although syringomyelia or multiple sclerosis was suspected, the diagnosis was uncertain and there was no further work-up. In May 2003, he presented to our hospital because of chest and back pain. A neurologic examination on admission revealed paraplegia in both lower extremities, pain and numbness at the left T6–8 region, and sensory disturbance below the T7 level. Deep tendon reflexes were absent, and Babinski sign was negative in the bilateral lower extremities. Difficulty in urination was also observed. MR images of the thoracic spine revealed a mass lesion in the spinal cord between the level of the T6 and T7 vertebral bodies with hyperintensity on T2WI and isointensity on T1WI, and heterogeneous enhancement on T1WI obtained after an intravenous injection of contrast medium, which indicated an intramedullary tumor (Fig 1B–G). Spinal cord atrophy was demonstrated at the upper and lower ends of the intramedullary tumor at the levels of T5 and T8 vertebral bodies (Fig 1H and -I), and the tumor showed a “string of beads”–like appearance. According to these findings, our preoperative diagnosis was an intraspinal germinoma. A T6–T7 laminectomy was performed. The thoracic portion of the cord was enlarged from T6 to T7 by an intramedullary tumor. There was no apparent spinal dissemination of the tumor. The tumor was completely resected.
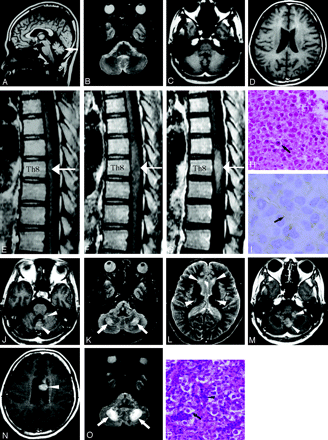
Patient 1. MR images of the thoracic spine and brain.
A, T2-weighted axial image of the thoracic spine in 1999 revealed an area of increased signal intensity changes in the spinal cord at the level of the T7 vertebral body (arrow). Enlargement of the spinal cord was not observed. Good sagittal MR images were not obtained, because of scoliosis.
B–G, Sagittal and coronal MR images of the thoracic spine in 2003 showed enlargement of the spinal cord between the level of T6 and T7 vertebral bodies, with hyperintensity on T2WI (B and E, arrows), isointensity on T1WI (C and F, arrows) and heterogeneous enhancement on T1WI obtained after an intravenous injection of contrast medium (D and G, arrows), which suggests an intramedullary tumor. Spinal cord atrophy was demonstrated at the upper and lower ends of the intramedullary tumor at the levels of T5 and T8 vertebral bodies on sagittal and coronal images (B–G, arrowheads). The tumor showed a “string of beads” appearance (B and E, asterisks).
H and I, Spinal cord atrophy was also confirmed on T2-weighted axial images at the levels of T5 and T8 vertebral bodies (arrowheads).
J, Photomicrograph of a specimen of the intraspinal tumor showed large round cells with clear cytoplasm and central nuclei (arrow) along fibrovascular septa. Small lymphocytes were not apparent (hematoxylin-eosin stain; original magnification, ×66).
K, Photomicrograph of a specimen of the intraspinal tumor showed that the cell membrane (arrow), which stains brown, was immunoreactive for PLAP. Cell membrane expression of PLAP was characteristic of germinoma (placental alkaline phosphatase stain; original magnification, ×132).
L and M, The left putamen was atrophic and showed high signal intensity change on T2WI (L, arrowhead). No contrast enhancement was observed on T1WI obtained after an intravenous injection of contrast medium (M, arrowhead). The presence of germinoma was suggested in the left putamen.
On histopathologic examination , microscopic inspection of specimens of the resected tumor revealed large round cells with clear cytoplasm and central nuclei along fibrovascular septa (Fig 1J). Although small lymphocytes were not apparent, the cell membrane was immunoreactive for placental alkaline phosphatase (PLAP; Fig 1K). The cells were negative for human chorionic gonadotropin (hCG) and alfa-fetoprotein in other immunohistochemical staining. Cell membrane expression of PLAP was characteristic of germinoma. Thus, the tumor was diagnosed conclusively as a germinoma. An increase in the serum hCG level (13.3 mU/mL; standard ≤3.0) was confirmed postoperatively.
Because the patient was a tall (180 cm) and slender man who had shown gonadal dysfunction, Klinefelter syndrome was suspected. Cytogenetic studies of peripheral blood lymphocytes revealed a 47XXY karyotype, and the diagnosis of Klinefelter syndrome was confirmed. MR images of the brain revealed atrophy of the left putamen, with increased signal intensity changes on T2WI (Fig 1L), which suggested germinoma. No contrast enhancement was observed after an intravenous injection of contrast medium (Fig 1M). CT of the chest and abdomen demonstrated no abnormalities in the mediastinum or the testes. Irradiation to the whole spine (24 Gy in total) and combined chemotherapy (carboplatin and etoposide) were administered after surgery, but neurologic findings remained stable. In June 2004, MR images of the brain showed atrophy of the left putamen without interval changes. In May 2005, there was no evidence of tumor recurrence in the spinal cord on MR images of the thoracic spine.
Case 2
Patient 2 was a 27-year-old, mentally retarded Japanese man. He had previously been diagnosed with Klinefelter syndrome (47XXY) on the basis of cytogenetic studies. In December 1989 (when he was 22 years old), he presented to our hospital because of a 10-month history of gait disturbance. A neurologic examination on admission revealed ataxic paraplegia in the bilateral lower extremities, positive Babinski sign in the left lower extremity, sensory disturbance below the T8 level and pain in the lower abdomen and thighs, mild dysarthria, saccadic eye movement, and downbeat nystagmus. Urinary dysfunction was not present.
MR images of the brain revealed cerebellar atrophy (Fig 2A) without abnormal signal intensity changes, which suggested the presence of tumors on MR imaging, though contrast medium was not given (Fig 2B–D). MR images of the thoracic spine demonstrated a slight enlargement of the spinal cord at the T8 level (Fig 2E), but definite diagnosis was not established. In March 1991, MR images of the thoracic spine demonstrated further enlargement of the lesion in the spinal cord between the level of the T7 and T9 vertebral bodies, with hyperintensity on T2WI and isointensity on T1WI and heterogeneous enhancement on T1WI obtained after an intravenous injection of contrast medium, which indicated an intramedullary tumor (Fig 2F and G).
Patient 2. MR images of the thoracic spine and brain. Because these images were obtained by using an old MR system with low magnetic field (0.5T), images were relatively poor in quality.
A, T1-weighted sagittal image of the brain in 1989 showed cerebellar atrophy (arrow).
B–D, No abnormal signal intensity changes suggesting the presence of tumors were detected on MR images, though contrast medium was not given.
E, T1-weighted sagittal image of the thoracic spine in 1989 demonstrated a slight enlargement of the spinal cord at the T8 level (arrow).
F and G, MR images of the thoracic spine in 1991 demonstrated further enlargement of the lesion in spinal cord between the level of T7 and T9 vertebral bodies, with hyperintensity on T2WI (not shown), isointensity on T1WI (F, arrow), and heterogeneous enhancement on T1WI obtained after an intravenous injection of contrast medium (G, arrow).
H, Photomicrograph of a specimen of the intraspinal tumor showed large round cells with clear cytoplasm and central nuclei (arrow). Small lymphocytes were not apparent (hematoxylin-eosin stain; original magnification, ×66).
I, Photomicrograph of a specimen of the intraspinal tumor showed that the cell membrane (arrow), which was stained brown slightly, was immunoreactive for PLAP. Cell membrane expression of PLAP was characteristic of germinoma (placental alkaline phosphatase stain; original magnification, ×132).
J, MR images of the brain in 1992 revealed slight contrast enhancement in the cerebellum on T1WI obtained after an intravenous injection of the contrast medium (arrowheads).
K and L, MR images of the brain in 1992 revealed lesions with increased signal intensity changes on T2WI in the corpus medullaris cerebelli (K, arrows), periventricular white matter around the anterior horn, and corticospinal tracts on both sides (L, arrowheads).
M and N, MR images of the brain in 1994 demonstrated multiple tumors in the cerebellum and the left lateral ventricle on T1WI after an intravenous injection of contrast medium (M and N, arrowheads).
O, MR images of the brain in 1994 demonstrated further enlargement of the lesions in the corpus medullaris cerebelli with increased signal intensity changes on T2WI (arrowheads). The lesions in the corpus medullaris cerebelli, periventricular white matter around anterior horn, and corticospinal tracts on both sides with increased signal intensity changes on T2WI remained stable.
P, Photomicrograph of a specimen of the tumor in the vermis showed 2 distinct cell types: large round cells with clear cytoplasm and central nuclei (arrow) were admixed with small lymphocytes (arrowhead) along fibrovascular septa, indicating a germinoma (hematoxylin-eosin stain; original magnification, ×66).
No abnormalities were detected in the mediastinum or testes by preoperative CT of the chest or ultrasonography. A T6–T10 laminectomy was performed. The thoracic portion of the cord exposed after incision of the dura was enlarged and was occupied from T7 to T9 by an intramedullary tumor. There was no apparent spinal dissemination of the tumor. Because of the difficulty of distinguishing between the tumor and the normal spinal cord, the tumor was partially resected. The specimen of the resected tumor was very small. On histopathologic examination, microscopic inspection of specimens of the resected tumor revealed large round cells with clear cytoplasm and central nuclei (Fig 2H). Small lymphocytes were not apparent. PLAP staining was not performed at that time.
The histopathologic diagnosis at that time had been “malignant glioma,” but this diagnosis was changed. Local irradiation (48 Gy in total) was administered after surgery. In August 1991, no enlargement of the spinal cord or contrast enhancement after an intravenous injection of contrast medium was observed on MR images of the thoracic spine. In November 1992, MR images of the brain revealed slight contrast enhancement in the cerebellum after an intravenous injection of the contrast medium (Fig 2J). Cerebellar atrophy remained stable. T2WI revealed lesions with increased signal intensity changes in the corpus medullaris cerebelli, periventricular white matter around the anterior horn, and corticospinal tracts on both sides (Fig 2K and -L). No contrast enhancement was observed in these areas.
In July 1994, MR images of the brain demonstrated multiple tumors in the cerebellum and the left lateral ventricle (Fig 2M and -N) and further enlargement of the lesions in the corpus medullaris cerebelli with increased signal intensity changes on T2WI (Fig 2O). The lesions in the periventricular white matter around the anterior horn and corticospinal tracts on both sides with increased signal intensity changes on T2WI remained stable.
An open biopsy of the vermis was performed. On histopathologic examination, microscopic inspection of the specimens of the tumor revealed that the tumor tissues were composed of 2 distinct cell types. Large round cells with clear cytoplasm and central nuclei were admixed with small lymphocytes along fibrovascular septa (Fig 2P). The tumor was diagnosed conclusively as a germinoma. Retrospectively, the histopathologic findings of the large round cells in the previously resected intraspinal tumor were similar to those of the tumor in the vermis. Cell membrane of the previously resected intraspinal tumor was confirmed to be immunoreactive for PLAP (Fig 2I). Thus, the intraspinal tumor was diagnosed as a germinoma. A slight increase in the serum hCG level (4.8 mU/mL) was also confirmed.
Combined chemotherapy (cisplatin and etoposide) was administered after surgery. In October 1994, MR images of the brain demonstrated prominent shrinkage of the residual tumors. In November 1994, there was no evidence of tumor recurrence in the spinal cord on MR images of the thoracic spine. In October 1995, however, MR images of the brain revealed enlargement of the residual tumors. In November 1998, the patient died of pneumonia at an outside hospital. Autopsy was not performed.
Discussion
Most CNS germ-cell tumors are sporadic. Klinefelter syndrome, however, is associated with an increased risk of both intracranial and mediastinal germ-cell tumorigenesis. According to Lantos et al,7 this susceptibility could reflect increased dosage of a chromosome X–associated gene, because CNS germ-cell tumors frequently exhibit extra copies of this chromosome. That the chronic elevation of serum gonadotropins characteristic of this disorder could also contribute to the development or progression of intracranial germ-cell tumors is suggested by the predilection of these lesions for peripubertal subjects and for the vicinity of diencephalic nuclei regulating gonadotropic activity. Intracranial germ-cell tumors have also been described in association with Down syndrome, which carries an increased risk of testicular germ-cell tumorigenesis. On record are isolated accounts of CNS germ-cell tumors arising in the setting of neurofibromatosis type I and involving siblings.
Various neoplasms such as a breast cancer, acute lymphoblastic leukemia, and both intracranial and mediastinal germ-cell tumors have been described as associated with Klinefelter syndrome.7,8 Germ-cell tumors in the CNS associated with Klinefelter syndrome, however, are extremely rare disorders.2 Including our patients, there were 12 reported patients with neoplasms in the central nervous system associated with Klinefelter syndrome (Table 1).2,6,9–16 Eleven patients had intracranial tumors, of which 9 were germ-cell tumors (8 germinomas and a single malignant mixed germ-cell tumor), one was lymphoma, and one was pilocytic astrocytoma. The 9 patients with intracranial germ-cell tumors ranged in age from 12 to 35 years (mean, 19.4 years), and 3 had tumors located in the pineal region, 3 in the hypothalamic region, one in the suprasellar region, one in the medulla oblongata, and one in the cerebellum and intraventricular region. There were only 3 patients with intraspinal tumors associated with Klinefelter syndrome, and these were diagnosed as germinomas in all 3 patients. The age of those patients with intraspinal germinomas ranged from 27 to 35 years (mean, 30.3 years).
Summary of previous cases of tumors in the central nervous system associated with Klinefelter syndrome
Whether these brain tumors and spinal cord tumors in our patients were primary or metastatic is the key question. We think the brain tumors and spinal cord tumors in our patients were synchronous; in other words, multifocal germ-cell tumors. Nagasawa et al reported a patient with intracranial and spinal germinomas occurring 4 years after spinal cord germinoma, and, according to the authors, germ cells could be present simultaneously in different locations in the CNS.17
Germinomas are often observed in young men, and extragonadal germ-cell tumors arise predominantly in midline structures of the body such as the mediastinum and retroperitoneum and intracranially the pineal and suprasellar regions. Intraspinal germinomas are very rare.18 To the best of our knowledge, including our patients only 17 cases of primary intraspinal germinoma have been reported (Table 2).5,6,17–29 The patients, who ranged in age from 5 to 35 years (mean, 25.4 years), were children and young adults. There were 11 male and 6 female patients. The tumors occupied the lower thoracic and the lumbar portions of the cord in 13 patients. The tumors occupied the cervical portions of the cord in 2 patients, and the upper thoracic portion of the cord in 2 patients. Thirteen of the 17 patients were examined with values of hCG in the serum or CSF, and increased values of hCG were observed in 7 of the 13 patients. Fourteen of the 17 patients were examined with MR imaging. The intramedullary tumors, which were accompanied by spinal cord enlargement, demonstrated isointensity or low signal intensity or slightly high signal intensity on T1WI. The 14 tumors shown on MR imaging appeared as low- and high-signal intensity changes on T2WI; they showed contrast enhancement after intravenous injection of contrast medium in all but one patient. The tumors contained cystic lesions in 3 patients. Cystic lesions are a nonspecific finding, though they are consistent with germinoma. Thus, the differential diagnosis from an astrocytoma and ependymoma can be difficult. Spinal cord atrophy above and below the tumor (“string of beads”–like appearance) observed in patient 1 may be an important diagnostic sign of intraspinal germinoma, though no previous studies reported spinal cord atrophy accompanied by intraspinal germinomas.
Summary of previous cases of primary intramedullary spinal cord germinoma
Intracranial germinomas, particularly in the basal ganglia, tend to cause ipsilateral hemicerebral atrophy in some cases.30–33 Germinomas cause degeneration and disappearance of ganglion cells and nerve fibers in the affected region,30 and atrophic changes of ipsilateral cerebral hemisphere might be due to secondary Wallerian degeneration.30–32 In patient 1, atrophy was present in the left basal ganglia without apparent tumors on T1WI after injection of contrast medium. According to Okamoto et al, atrophy of the basal ganglia could be a recognizable initial and diagnostic MR imaging sign of germinoma in the basal ganglia.33
To the best of our knowledge, only one case with cerebellar atrophy accompanied by intracranial germinoma was reported.34 According to this case report, cerebellar ataxia was ascribed to the “remote effect” of germinoma; however, an exact cause was not described. In patient 1, atrophy was present in the left basal ganglia without apparent tumors on T1WI after injection of contrast medium. In patient 2, atrophy was present in the cerebellum without abnormal signal intensity changes, which suggests the presence of tumors, though contrast medium was not given. Therefore, it was unlikely that germinoma directly destroyed ganglion cells and nerve fibers. We suspect that intracranial very small germinoma not detected on MR images secreted “toxin” that caused degeneration of nerve fibers and degeneration and disappearance of ganglion cells. In patient 2, T2WI showed lesions with T2 elongation in the corpus medullaris cerebelli, periventricular white matter around the anterior horn and corticospinal tracts on both sides. We think that the areas might be due to demyelination caused by a toxin. In case 1, we speculate that spinal cord atrophy was also caused by a toxin secreted by spinal cord germinoma. In other tumors—such as astrocytoma, ependymoma, and hemangioblastoma—toxin was not secreted; therefore, atrophy was not present.
If an intraspinal tumor is detected in a child or a young adult, germinoma should be included in the differential diagnosis. Particularly in a patient with Klinefelter syndrome, a relatively long course of the history, spinal cord atrophy above and below the tumor (string of beads–like appearance), atrophy of the brain, or an increase in the serum hCG level, the possibility of germinoma may increase. In the 2 patients presented here, attention to these findings was considered to have led to the correct diagnosis of germinoma. Also, if an intraspinal germinoma was suspected, MR imaging of the brain was considered to be necessary even in asymptomatic patients.
References
- Received June 10, 2005.
- Accepted after revision July 19, 2005.
- Copyright © American Society of Neuroradiology