Abstract
BACKGROUND AND PURPOSE: This study assesses the cytotoxicity of hyperosmolar mannitol on human endothelial and meningioma cells in vitro and summarizes the initial clinical experience of using mannitol as a liquid tumor embolization agent.
METHODS: Human umbilical vein endothelial cells and primary meningioma cells from surgical specimens were treated with 300, 600, 900, and 1200 mOsm of mannitol, mannitol and iohexol mixture, saline, and iohexol alone. Cell death was evaluated with a Live/Dead kit and quantified with thymidine incorporation. From 1998 to 2004, 23 patients with meningioma were treated with mannitol and 31 patients were treated with polyvinyl alcohol (PVA) particles alone. Angiographic results, procedural complications, intraoperative observation, and estimated blood loss during surgical resection were retrospectively evaluated.
RESULTS: Minimal endothelial cell death was seen after incubation with 300 mOsm of mannitol for 15 minutes, but 43 ± 2% of endothelial cells were damaged by 1200 mOsm of mannitol after 30 minutes. Five meningioma cell lines exhibited significant cell death (22 ± 2%; P < .05) after incubation with mannitol. Satisfactory angiographic results were obtained in all 23 patients. Tumor necrosis was observed intraoperatively and confirmed pathologically. There was no significant difference in estimated blood loss between mannitol- and PVA-embolized patients (407 ± 64 mL vs 381 ± 50 mL; P > .75).
CONCLUSION: High concentration of mannitol can injure endothelial cells and meningioma cells in a short period of time. It is feasible to use mannitol as a liquid embolic agent to treat meningioma.
Chemoembolization has emerged as an effective therapy for certain types of unresectable tumors (1, 2). Palliative or adjunctive chemoembolization of brain tumor has also been reported, but it requires a wide safety margin because nontargeted embolization in the brain can cause stroke (3–5). Particulate agents are commonly used to embolize external carotid branches that supply head and neck tumors and meningiomas (6–9). Liquid agents such as n-butylcyanoacrylate and ethylene vinyl alcohol copolymer, which have been used for embolization of arteriovenous malformations, are more suitable for vascular pedicles with rapid flow rate and do not penetrate well into small tumor vessels. Alcohol has also been used for embolization of venous malformations, dural arteriovenous fistulas, and head and neck tumors (10), but its feasibility and safety for brain tumor embolization has not been fully assessed. In this study, we went from bench to bedside to assess the feasibility of using hyperosmolar mannitol as a liquid chemoembolization agent.
Hyperosmotic materials have been used clinically as embolic agents to treat tumors (11–14). Hyperosmotic cell stress not only damages cells physically, but also induces apoptosis. Mechanisms proposed include activation of proinflammatory cytokine production (15) and activation of mitogen-activated protein kinases (16) including stress kinases p38 and c-Jun n-terminal kinase (17). Mannitol, a six-carbon nonmetabolizable sugar that is impermeable to cells, is widely used clinically for acute and subacute reduction of brain edema. Intravenous administration of mannitol reduces intracranial pressure and improves cerebral blood flow (18, 19). Mannitol is also a free radical scavenger and, as such, may have neuroprotective properties (20). Mannitol in hyperosmolar concentrations, however, is cytotoxic and has been shown to induce apoptosis in bovine endothelial cells in a dose-dependent manner, accompanied by activation of tyrosine and stress kinases, phosphorylation of fecal adhesion kinase and paxillin, and elevation of intracellular free calcium (21). Damaged endothelial cells can active the coagulation pathway, leading to intravascular thrombosis (22–26). A relatively low concentration of 450 mOsm of mannitol was used in the previous report (21) and the time required for induction of apoptosis was 6 hours, too long to use it clinically as an embolic agent.
On the basis of these data, we hypothesized that higher concentration of mannitol may damage endothelial cells in shorter period of time. After confirmatory laboratory study, we began by using mannitol as a liquid embolic agent to treat intracranial meningiomas. This report presents both laboratory and clinical data to support the use of mannitol as a liquid embolic agent for meningioma treatment.
Methods
Cell Cultures
To support the hypothesis that mannitol can damage endothelial and tumor cells within a reasonable time frame, cell culture experiments were carried out on human umbilical vein endothelial cells (HUVECs) and primary cultures of surgically resected meningiomas. HUVECs were prepared as described elsewhere (27) with collagenase digestion of fresh human umbilical cords. Only the first five passages of cells were used in the following experiments. They were plated at a density of 200/mm2 in endothelial growth medium (EGM) (Clonetics, Baltimore, MD) on eight-well plastic chamber slides or 96-well plates (Nalge Nunc International, Naperville, IL) precoated with 2% gelatin. The cells had typical cobblestone appearance and usually reached 80% confluence after overnight growth. To obtain primary meningioma cells, surgical specimens were minced to small pieces, dissociated with trypsin, and plated on polylysine-coated flasks in high glucose Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Rochester, NY). The morphology of the cultured meningioma cells varied from large, flat, and multinucleated cells to small spindle cells. After several passages, the cells were frozen in liquid nitrogen as stocks. Before the cytotoxicity experiment, the cells were thawed and recovered in DMEM containing low glucose. They were plated on eight-well plastic chamber slides or 96-well plates at an attenuation of 200/mm2 and allowed to reach confluence before mannitol treatment.
The treatment solutions were prepared by diluting 25% mannitol (Abbott Laboratories, Abbott Park, IL) in water to create a mannitol gradient of 300, 600, 900 and 1200 mOsm. The 25% mannitol was also mixed with iohexol 300 (Nycomed, Princeton, NJ) at a 2:1 ratio to give an osmolality of 1015 mOsm, including 915 mOsm from mannitol. After removal of the culture medium, primary cultures of HUVECs were treated with the mannitol gradient, iohexol, mannitol/iohexol mixture (2:1), and normal saline for 15 or 30 minutes. The meningioma cells were treated with 300 and 600 mOsm of mannitol, iohexol, and diluted mannitol/iohexol mixture (1:1 with medium) for 1 hour. The lower concentrations of mannitol were used to take into consideration the dilution of mannitol by extracellular fluid before it reached meningioma cells. Because the clearance of interstitial mannitol was expected to be much slower than intravascular mannitol, the meningioma cells were also treated for longer time.
To demonstrate endothelial damage after mannitol treatment, the cells were stained with Live/Dead kit (Molecular Probe, Eugene, OR). The Live/Dead kit had two components: ethidium bromide dimer (2 mmol/L) and Calcein AM (4 mmol/L). Ethidium bromide, an orange fluorescent dye, was excluded by live cells with intact cell membrane but was picked up by the nuclei of dead cells, which had compromised cell membranes. Calcein AM, which was not fluorescent, diffused through the cell membrane of live cells and was catalyzed to a green-fluorescent form in an energy-dependent manner, which was trapped within the cytoplasm. Therefore, the live cells appeared green under fluorescent microscope and the dead cells were depicted by orange nuclei stain. This method was not suitable for quantification of cell injury because many damaged cells detached easily from the plate and were lost in washing solution before observation under fluorescent microscope.
To quantify the cytotoxic effect of mannitol, HUVECs and meningioma cells were plated on 96-well plates at 200/mm2 and labeled with 6 μci/mL of 3H-thymidine (NEN, Boston, MA) for 48 hours. After treatment with mannitol, the cells were washed vigorously with Hanks solution containing 5% bovine serum albumin. Dead or damaged cells detached from the plates, releasing their thymidine count into the supernatant. The remaining live cells that attached to the plates were dissociated with trypsin. Both supernatant and cellular component were counted with scintillation counter (DuPont, Wilmington, DE). The percentages of supernatant count in total 3H-thymidine incorporation in triplicate experiments were averaged and normalized to saline control to obtain cell death rate. Because acute damage of endothelial cells could lead to thrombosis, cell death of HUVECs was assessed immediately after treatment. By contrast, meningioma cells were expected to die over longer period of time. They were treated for 1 hour, and cell death was assessed over a 48-hour period.
To determine whether programmed cell death played an important role after mannitol treatment, the meningioma cells were stained with TUNEL reaction kit (ApopTag; Intergen, Purchase, NY). The TUNEL reaction worked by labeling the broken ends of DNA in apoptotic cells with digoxigenin-labeled deoxyuracil triphosphate, which was subsequently detected by using a peroxidase conjugated antidigoxigenin antibody. After 1-hour treatment with 900 mOsm of mannitol, culture medium, or 900 mOsm hypertonic saline, the treatment solutions were removed and the meningioma cells were allowed to recover in normal culture medium for 6 hours. The cells were then fixed with 4% paraformaldehyde (Sigma/Aldrich, Grand Island, NY) and stained with the TUNEL reaction kit according to the manufacturer’s instructions.
Clinical Study
From July 1998 to June 2004, mannitol was used as an off-label embolization agent in 23 patients with meningioma at our institution. The average age of these patients, including five men and 18 women, was 59 ± 4 years, ranging from 22 to 84 years (Table 1). Eighteen patients underwent tumor resection after an average interval of 2 days. Five patients received embolization as a palliative treatment to alleviate worsening mass effect and were discharged from the hospital without surgery.
Patients with meningioma treated with preoperative embolization
After diagnostic angiograms demonstrated tumor blush, the meningeal arteries that supplied the tumor were superselectively cannulated with a braided microcatheter, usually to a position of near flow arrest. If near flow arrest could not be achieved, or there was rapid washout of contrast stain in the tumor vascular bed, a small amount (∼1–2 mL) of diluted 150–250-μ polyvinyl alcohol (PVA) particles was infused into the feeding vessels to reduce flow, thus maintaining mannitol concentration and increasing contact time between mannitol and tumor vessels. Under digital subtraction fluoroscopy, 20–60 mL of mannitol/iohexol mixture (2:1 ratio) was continuously infused over 15–30 minutes into the feeding artery until contrast stagnation was evident. To reduce the risk of intratumoral hemorrhage after embolization, proximal occlusion of the meningeal arteries were achieved with Gelfoam or coils if the patient was scheduled for surgery.
In the same time period, 31 patients were treated with conventional PVA particles. Mannitol was planned to be used in four of these patients, but tumor blush was obliterated by a small amount of PVA particles before mannitol infusion started. Twenty-seven of these patients were treated by a physician who did not participate in the off-label use of mannitol.
We retrospectively evaluated the procedural complications, estimated blood loss during surgery, and tumor necrosis in pathologic specimen in this case series and compared the estimated blood loss with that of patients treated with conventional PCA particles. The operating neurosurgeons were also surveyed immediately after tumor resection for their satisfaction with tumor embolization. Estimated blood loss was obtained from the operative reports and compared between patients treated with mannitol and conventional PVA particles by using the Student t test. Estimated blood loss was not recorded for four patients in the mannitol group and six patients in the PVA group. Tumor necrosis was assessed by reviewing the pathology reports. This retrospective study received approval by Institutional Review Board of Columbia University.
Results
Cytotoxic Effect of Mannitol on Endothelial Cells
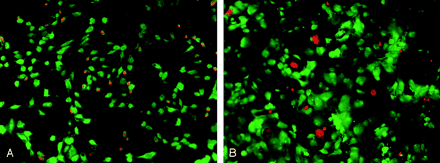
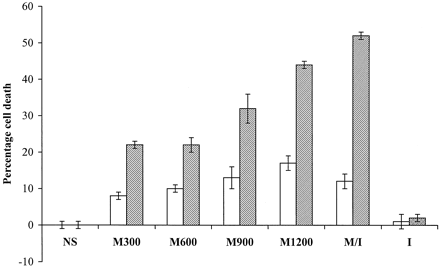
The cytotoxic effect of mannitol on endothelial cells was clearly demonstrated with the Live/Dead kit (Fig 1). Almost all endothelial cells shrunk after mannitol exposure, and many of them picked up the ethidium bromide dimmer, which suggests compromised cell membrane. Dose- and time-dependent cytotoxic effects of mannitol were observed on cultured HUVECs in the thymidine labeling experiment (Fig 2). Minimal loss of HUVECs was seen with 300, 600 and 900 mOsm of mannitol for 15 minutes. More cells were killed with 1200 mOsm of mannitol. Significantly more cytotoxicity occurred after 30 minutes of treatment for all concentrations. The cell death rate increased with concentration, reaching 44 ± 1% with 1200 mOsm at 30 minutes. Mannitol mixed with iohexol at 2 : 1 (915 mOsm of mannitol plus 100 mOsm of Iohexol) had similar effect, killing 52 ± 1% of endothelial cells at 30 minutes. Iohexol itself exhibited no significant cytotoxic effect after 30 minutes of incubation. The higher killing rate of mannitol/iohexol mixture than mannitol alone may be the result of the higher density of mannitol/iohexol mixture, which could have facilitated the detachment of lightly injured cells by buoyancy.
Cytotoxicity of mannitol on endothelial and meningioma cells.
A, HUVECs stained with LIVE/DEAD kit. After treatment with 1200 mOsm for 15 minutes, almost all endothelial cells shrank and lost their typical cobblestone morphology. Many of them detached from the culture plate. Orange nuclei depicting dead cells can be seen on the remaining cells.
B, Meningioma cells stained with LIVE/DEAD kit. Similar cytotoxic effect is seen in the meningioma culture after incubation with 600 mOsm for 1 hour.
Dose- and time-dependent cytotoxic effect of mannitol on endothelial cells. The kill rates of endothelial cells by various concentrations of mannitol after 15- (white bars) and 30-minute (gray or hatched bars) incubation are shown in this graph. NS, normal saline; M300, 300 mOsm mannitol; M600, 600 mOsm mannitol; M900, 900 mOSm mannitol; M1200, 1200 mOsm mannitol; M/I, 25% mannitol and iohexol mixed at 2 : 1 ratio; I, iohexol alone. A small portion of cell death (8–10 ± 1%) is caused by <900 mOsm mannitol after 15 minutes of incubation. As much as 17 ± 2% of endothelial cells die after 15 minutes of incubation with 1200 mOsm mannitol. When incubated for 30 minutes, the cell death rates are significantly higher for all concentrations of mannitol (P < .01 by t test). Mannitol and iohexol mixture has the highest cell death rate, at 52 ± 1%. Normal saline and iohexol alone have little effect on survival of endothelial cells. The error bars depict standard errors.
Cytotoxic Effect on Meningioma Cells
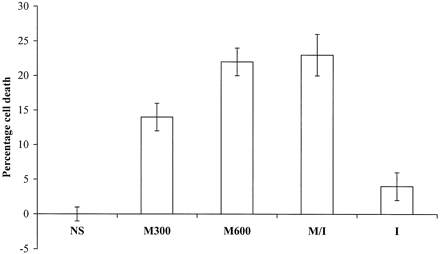
Dose-dependent cytotoxic effect of mannitol was observed in all five meningioma cell lines (Fig 3). On average, 300 mOsm of mannitol killed 14 ± 2% of meningioma cells in 1 hour. The cell death rates for 600 mOsm of mannitol and mannitol/iohexol mixture were similar, at 22 ± 2% and 23 ± 3%, respectively, comparable to the death rate of endothelial cells treated with 600 mOsm of mannitol for 30 minutes.
Cytotoxic effect of mannitol on five meningioma cell lines. The average kill rates of five meningioma cell lines by 300 mOsm (M300) of mannitol, 600 mOsm (M600), of mannitol and mannitol/iohexol 2:1 mixture (M/I) are shown in this graph. The cell death rates are 22 ± 2% and 23 ± 3% for 600 mOsm of mannitol and mannitol and iohexol mixture, respectively. Little cytotoxic effect is seen for normalsaline (NS) and iohexol alone (I).
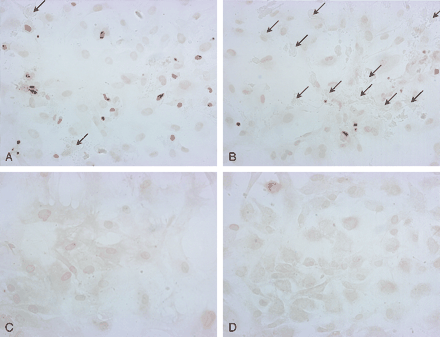
We observed both lytic and apoptotic cell death after treatment with mannitol. Some cells disintegrated, leaving a “ghost” on the culture dish; others underwent programmed cell death, becoming shrunken with condensed nuclei that were strongly positive for TUNEL reaction (Fig 4A and B). More apoptotic cells were observed in cell lines with faster proliferation rate. No significant lytic cell death or apoptosis was seen in control samples or hypertonic saline. The high cytotoxicity of mannitol compared to the same osmolar of hypertonic saline supported other mechanisms of cell death in addition to osmotic pressure.
Apoptotic and lytic cell death in meningioma cells. After 1-hour treatment of meningioma cell lines with 900 mOsm of mannitol (A and B), normal saline (C), and 900 mOsm hypertonic saline (D), the cells are reacted with TUNEL reagents to detect programmed cell death. A rapidly proliferating meningioma cell line (A) demonstrates frequent apoptotic cells that are stained brown by the TUNEL reagents. There are very few ghosts of dissolved cells, which are marked by the arrows. More lytic (arrows) cell death than apoptotic cell death (brown cells) occurs in a slow-growing meningioma line (B). Normal saline and hypertonic saline controls reveal no significant cell death.
Clinical Response to Mannitol Embolization
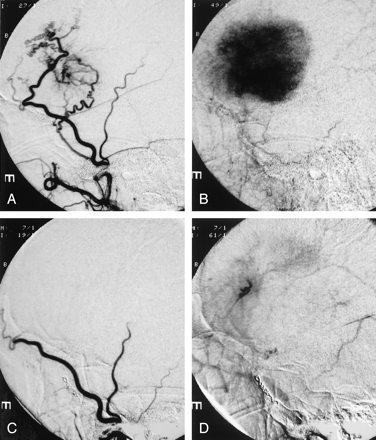
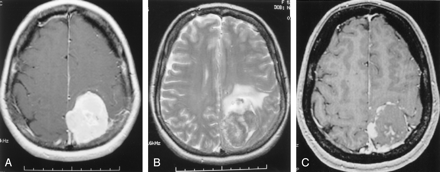
Flow arrest was achieved in the target tumor vessels in all patients embolized with mannitol. The amount of PVA used was much smaller than the amount used without mannitol: 1–2 mL of diluted PVA particles versus 10–15 mL of similarly diluted PVA particles. In this case series, we were able to catheterize the origin of the meningohypophyseal trunk and infuse mannitol to treat tentorial or petrous meningeoma, which often derived blood supply from the cavernous carotid artery. We also succeeded by using mannitol to embolize middle meningeal artery branches with internal carotid or ophthalmic artery collaterals, because mannitol was rapidly diluted below the threshold of cytotoxicity by blood flow in internal carotid or ophthalmic artery when it passed through the collateral channels. PVA embolization would have posed significant risk of stroke in the above two situations. Figure 5 shows a typical example of mannitol embolization. A large homogeneously enhancing left frontal meningioma caused progressive right hemiparesis and aphasia in this patient. The tumor derived exuberant blood supply from left middle meningeal artery and exhibited signature tumor blush in the capillary phase. Mannitol infusion resulted in vascular stasis and >90% reduction of tumor blush. MR imaging obtained before and after tumor embolization showed elimination of contrast enhancement in the center of a left parietal meningioma, which suggests tumor necrosis (Fig 6).
Mannitol embolization of a large frontal meningioma.
A, Tumor vessels coming off the left middle meningeal artery are shown by this early arterial phase of left external carotid angiogram.
B, The capillary phase of the same angiogram reveals the typical tumor blush in the meningioma.
C, Postembolization angiogram of the left external carotid artery shows lack of filling of the tumor vessels in the early arterial phase.
D, Most of the tumor blush has been eliminated and contrast stagnation is seen in one of the middle meningeal branch in the delayed phase.
MR imaging changes after embolization.
A, Before embolization, T1-weighted axial image of the brain with gadolinium reveals a large left parietal extraaxial homogenously enhancing tumor, consistent with meningioma.
B, T2-weighted axial image demonstrating significant mass effect and parenchymal edema.
C, After embolization, only the periphery of the tumor shows enhancement on this postcontrast T1 axial image. The center of the tumor becomes necrotic.
The operating neurosurgeons (J.B., M.S.) were satisfied with embolization results and observed necrotic tumors in all cases. The estimated blood loss ranged from 100 to 800 mL, with the mean of 407 ± 64 mL, which was not significantly different from the blood loss in patients who received conventional PVA embolization (381 ± 50 mL; P > .75). The average size of the tumors was 6.3 ± 0.4 cm for the mannitol groups and 6.6 ± 0.4 cm for the PVA group. Nine of 23 tumors in the mannitol group involved the skull base, similar to 11 of 31 tumors in the PVA group. The frequency of necrosis in tumor specimen was similar among the mannitol and PVA treated groups (47% vs. 45%). This frequency, however, may not represent the tumorocidal effect of embolization because only small pieces of the tumor were sent to pathology. One specimen embolized with mannitol also showed large number of apoptotic cells.
One minor complication unrelated to the use of mannitol occurred during the 23 procedures of mannitol embolization. The guidewire perforated a distal branch of the middle meningeal artery, resulting in a small asymptomatic epidural hematoma, which did not require treatment. The patient had uneventful surgery 1 day later. None of the patients developed new neurologic symptoms after embolization. One patient had transient worsening of preexisting symptoms after embolization. He presented for palliative therapy for rapid visual loss from a large recurrent sphenoid meningioma. He became blind after mannitol embolization but recovered his vision to baseline level after intravenous mannitol treatment. All four patients who received palliative treatment had improved symptoms at discharge.
Discussion
Two in vitro experiments demonstrate that a high concentration of mannitol injures endothelium in a short period of time. It has long been known that mannitol disrupts the blood-brain barrier, presumably by opening tight junctions between endothelial cells (28). Mannitol has thus been used along with chemotherapeutic agents to increase brain penetration (29–31). The high concentrations of mannitol used in our experiments may further widen the gap between endothelial cells, resulting in cell detachment, directly damage the endothelial cells by its osmotic force, or activate endothelial gene expression to promote programmed cell death. All these mechanisms may happen at the same time, leading to thrombosis (32). Endothelial detachment or cell death will expose underlying collagen in the basement membrane, which is strongly thrombogenic. Endothelial activation results in the recruitment of inflammatory cells and platelet, which will further promote clot formation. As a result, the cell detachment and cell death observed in the in vitro experiments strongly suggest that high concentrations of mannitol can be used to promote intravascular thrombosis.
The dose- and time-dependent cytotoxicity of mannitol on endothelial cells allows easy control of embolization. Only the target vessels that are selectively catheterized are likely to receive mannitol in sufficiently high concentrations to cause significant endothelial injury. The small amount of mannitol that is infused or back-flushed into normal vessels will be diluted and rapidly washed out without any detrimental effect on endothelial cells. Mannitol that diffuses to normal tissue from tumor interstitium is likely to be diluted and removed by normal blood flow to harm normal tissue. Low concentration of mannitol, used at the intravenous dose (∼300 mOSm), is safe and may have a neuroprotective effect from reduced brain edema and its free-radical scavenger properties (20). Therefore, embolization with mannitol is expected to have a wide safety margin, which is essential when embolizing tumor vessels coming off the internal carotid artery.
Our clinical experience demonstrates the feasibility of using mannitol as a liquid embolic agent for tumor treatment. Fifteen minutes of contact time with high concentration of mannitol is required to cause intravascular thrombosis. To prolong the contact time between high concentrations of mannitol and endothelial cells in the tumor vessels, a small amount of PVA can be used in most cases to slow blood flow in tumor vessels. Although mannitol infusion is also effective without PVA, rapid infusion of a large amount of mannitol would be required if there is significant arteriovenous shunting through the tumor. Improvement in four patients who received mannitol embolization as a palliative therapy without surgery suggests that mannitol embolization may be helpful for patients with unresectable tumors. Direct cytotoxicity of mannitol on tumor cells may improve the efficacy of embolization. Although all patients appeared well embolized at the time of surgery, the duration of vascular thrombosis after mannitol embolization is currently unknown. Damaged vessels may be repaired and recanalized over time. Repeat embolization may be performed through the same vascular pedicles after recanalization.
Most of the infused mannitol flows through the tumor vascular bed and extracts fluid from the tumor before vascular thrombosis takes place. Although hyperosmolar mannitol in the tumor interstitium may draw fluid from surrounding tissue to cause tumor swelling, devascularization may prevent the rapid shift of fluid into the tumor. In this case series, only one patient had transient deterioration after embolization, which suggests a low risk of tumor swelling after mannitol embolization.
The direct cytotoxic effect of mannitol on meningioma cells suggests that it may also be used as a chemotherapeutic agent. The in vitro study suggests that mannitol can cause both programmed cell death and lytic cell death in meningioma cells. Although the cell death rate is not very high in cell culture, the cytotoxicity is likely augmented by devascularization of the tumor. The presence of necrotic and apoptotic cells in surgical specimens support the toxic effect of mannitol on tumor cells.
Although the initial clinical results are encouraging, this study is limited by the lack of control group to establish the efficacy of mannitol embolization. Only a few patients had repeat MR imaging before surgical resection. Future study with serial imaging follow-up is required to answer the questions of tumor swelling and necrosis after embolization.
Conclusion
High concentrations of mannitol can injure endothelial cells and meningioma cells in a short period of time. Embolization of meningioma with mannitol is feasible and not associated with any unexpected adverse event in this case series.
Acknowledgments
We are grateful to the Weitzman family, who provided a grant to support this research.
Footnotes
Drs. Feng and Kiernitz made equal contribution to this article.
References
- Received April 2, 2004.
- Accepted after revision October 27, 2004.
- Copyright © American Society of Neuroradiology