Abstract
BACKGROUND AND PURPOSE: Histopathologic studies indicate that aneurysms treated with Guglielmi detachable coils (GDCs) have avascular centers with fibrosis mostly at the aneurysm periphery. We hypothesized that vascular endothelial growth factor (VEGF) released from a coil promotes clot organization, hyperplasia, and endothelial proliferation to facilitate closure of the aneurysm neck.
METHODS: GDC segments were inserted into ligated common carotid arteries (CCAs) of adult male rats for 14 days. Coil segments (4-mm) were unmodified, modified with type I collagen (2.4 mg/mL), or modified with type I collagen and recombinant human VEGF-165 (rhVEGF; 500 μg/mL). CCA segments were harvested and coils removed for scanning electron microscopy (SEM).
RESULTS: Collagen/rhVEGF coils (n = 11) resulted in marked reductions in CCA lumen area (0.03 mm2) compared with coils (n = 9, 0.21 mm2, P < .001) and collagen coils (n = 5, 0.13 mm2, P < .001). Collagen/rhVEGF coils (n = 11) also resulted in marked reductions in CCA diameter (0.19 mm) compared with coils (n = 9, 0.50 mm, P < .001) and collagen coils (n = 5, 0.40 mm, P < .001). Wall thickness was greater for the collagen/rhVEGF coil segments (0.22 mm) compared with coils (0.09 mm, P < .001), and the collagen coils (0.15 mm, P = .06). CCA segments containing collagen/rhVEGF coils also displayed Factor VIII positivity and were completely encapsulated in fibrotic tissue, while the unmodified and collagen coils were essentially smooth, as seen by SEM.
CONCLUSION: These results suggest that rhVEGF may be beneficial in promoting endothelialization, clot organization, and tissue integration of the coils. This is the first study to hypothesize that rhVEGF may be useful as a surface modification to GDCs for enhancing their therapeutic effects in the treatment of cerebral aneurysms.
Guglielmi detachable coils (GDCs) have been widely accepted in the treatment of intracranial aneurysms. Although they have been used successfully in a subgroup of aneurysm patients, platinum microcoils are relatively biologically inactive. Enhancement of their potential to cause thrombosis, clot organization, and eventual aneurysm neck closure may lead to improved therapeutic outcomes. Hence, there has been recent interest in modifying the surface of platinum GDCs to promote thrombosis and endothelial cell proliferation, and thus reduce the time necessary for aneurysm occlusion (1–7). The coils have been modified by coating the GDC surface with extracellular matrix proteins (collagen, laminin, fibronectin), non-biodegradable polymers (polyesters, polyurethanes), and fibroblasts, as well as ion impregnation of the platinum.
Vascular endothelial growth factor (VEGF) is a heparin-binding glycoprotein that also functions as a vascular permeability factor (8, 9). VEGF is produced by macrophages, endothelial cells, and smooth muscle cells (9) and is currently under intense investigation for its therapeutic effectiveness in elevating collateral flow in areas of myocardial ischemia. In the present study, we hypothesized that it is difficult for larger aneurysms to completely occlude after GDC therapy because the coils alone do not provide enough mechanical or biological support to promote neck closure. Also, large clots within coiled aneurysms may lack sufficient oxygen transfer and become ischemic or necrotic. Therefore, an angiogenesis-promoting factor such as VEGF may enhance fibrosis and subsequent aneurysm obliteration in a similar hemodynamic environment. The purpose of this investigation was to determine the feasibility of using platinum coils coated with recombinant human VEGF (rhVEGF) in an in vivo model of arterial stasis with arterial pressurization.
Methods
Coil Preparation
Under sterile conditions, Guglielmi detachable T-10 Coils (Target Therapeutics, Freemont, CA) were sectioned into 4-mm segments. The T-10 GDC is 0.2 mm (0.008 inches) in diameter with a cross-sectional area of 0.049 mm2. Segments were either unmodified (coil), coated with collagen (coil + collagen), or coated with collagen containing rhVEGF (coil + collagen/rhVEGF). Coil segments were coated with purified, pepsin-solubilized, bovine dermal Type I collagen (3.0 mg/mL) dissolved in 0.012 N HCl (Vitrogen, Cohesion Technologies, Palo Alto, CA). A collagen solution was made from 800 μL of this collagen solution, 100 μL of 0.1 N NaOH, and 100 μL of 10× phosphate buffered saline (Dulbecco's Phosphate Buffered Saline; Gibco BRL, Grand Island, NY) and thoroughly mixed (pH = 6.8). Coil segments were immersed in the solution (2.4 mg/mL collagen) and incubated at 37°C for 1 hour to allow for polymerization, and then air-dried in a sterile laminar flow hood for 1 hour. Immediately after drying, coils were maintained in a sterile environment and inserted into the ligated common carotid artery (CCA) of the rat.
For VEGF coating, an aliquot of 100 μL of 5 mg/mL rhVEGF (rhVEGF-165; Genentech, San Francisco, CA) was added to 900 μL of the collagen solution and mixed thoroughly for a final concentration of 500 μg/mL (pH = 6.9). Coil segments were immersed in the collagen/rhVEGF solution and incubated at 37°C for 1 hour to allow for polymerization, after which the coils were then air-dried in a sterile laminar flow hood for 1 hour. Immediately after they were dried, the coils were maintained in a sterile environment and inserted into the CCA of the rat.
Animal Surgery and Coil Placement
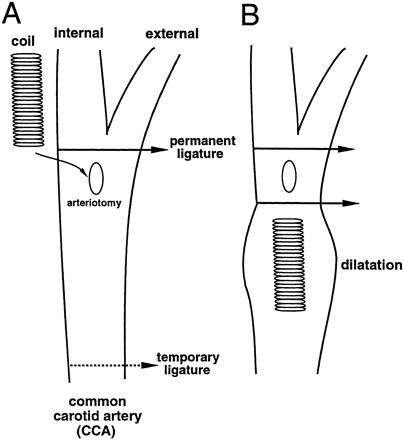
All procedures were approved by the University of Pennsylvania Regulatory Affairs Committee. Sprague-Dawley rats (375–425 g) were given access to food and water ad libitum before anesthesia was induced by an intraperitoneal injection of 60 mg/kg sodium pentobarbital. After induction of anesthesia, the animals were kept on a heating pad and maintained at 37°C for the entire procedure and immediate recovery period. In the supine position, a right paramedian incision was made from the angle of the mandible to the area of the mid-clavicle. The superficial facial and muscle layers were separated with blunt dissection until the carotid bundle was visualized. The CCA was skeletonized and a permanent ligature was placed proximal to the CCA bifurcation and a temporary ligature was placed 1 cm distal to the origin of the CCA (Fig 1A). After proximal control of the CCA was obtained with the temporary ligature, a small arteriotomy was made 2 mm proximal to the distal ligature. A coil segment was inserted into the CCA and a new ligature was placed just distal to the arteriotomy to exclude it from the circulation (Fig 1B). The temporary ligature was released to re-establish blood flow in the carotid segment. The operative field was inspected to assure that the final ligature maintained hemostasis and that the coil was secure in the newly created blind-ended sac (Fig 1B). The wound was closed and the animals were returned to their cages and allowed to recover for 14 days. Marked vasodilation proximal to the second permanent ligature was again noted upon removal of the temporary ligature.
A, Exposure of the CCA showing the temporary proximal and permanent distal ligatures.
B, The coil has been inserted into the CCA, a permanent ligature has been placed proximal to the arteriotomy, and the temporary ligature has been released.
Vessel and Coil Harvest
The animals were given access to food and water ad libitum for the next 14 days and then the coils were harvested from the CCA. Anesthesia was induced by an intraperitoneal injection of 60 mg/kg sodium pentobarbital, and the animals were sacrificed by an intracardiac injection of 60 mg/kg sodium pentobarbital. The previous incision was opened and the CCA exposed. The segment of CCA containing the coil was removed (approximately 10 mm in length) and sectioned in half and the coil segment was carefully removed. The coil and CCA segments were placed into formalin for preservation. In seven of the animals, the contralateral CCA was resected and preserved in formalin for section. These specimens were used as CCA contralateral controls.
Histopathology
Formalin-fixed CCA segments were embedded in paraffin and sectioned at a thickness of 6 μm. Each specimen was stained with hematoxylin and eosin (H&E) according to standard protocols. Specimens with fibrotic material within the lumen underwent immunohistochemical analysis with antibodies to Factor VIII (Accurate Chemicals, Westbury, NY) to assess the presence of this endothelial antigen. Coil segments were preserved in formalin and visualized by scanning electron microscopy (SEM). For semi-quantitative pathologic scoring, a single observer, who was blinded to the coil modification groups, evaluated histopathlogic sections. Evaluations were based on grade of intimal proliferation (0–3), percent of intimal occlusion (%), and grade of fibrosis (0–3).
Histopathologic sections of CCA sections were also viewed using a microscope (Axiovert 135, Carl Zeiss, Thornwood, NJ) and digitized using a calibrated CCD camera (Hamamatsu Phototonics, Inc., Bridgewater, NJ). Digitized images were captured with a frame-grabber on a Dell workstation (Dell Computers, Round Rock, TX). On the workstation, digitized images were analyzed using Image Analysis software (UTHSCSA Image Tool, San Antonio, TX) that allowed for quantitative measurements of the lumen area, circumference, and wall thickness. Lumen area was calculated by direct measurement of the perimeter of the “patent” lumen (the hole). The perimeter remained, for the most part, unaffected by distortion of the tissue upon fixation. The area occupied by the removed coil contributed to this “patent” lumen diameter. Given the large number of replicates in each group, it was unexpected that a handling artifact would alter the geometry of all six samples in a single group in the same way.
Each set of data was expressed as a mean ± standard deviation for the number of vessels analyzed in each set. A two-tailed t test was used to determine the statistical difference between treatment groups with Bonferroni correction for multiple comparisons to the CCA with unmodified coil.
Results
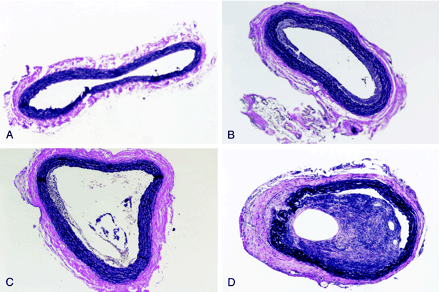
A total of 25 procedures were performed, with 31 arterial segments harvested and grouped into control (n = 6), coil (n = 9), coil + collagen (n = 5), and coil + collagen/rhVEGF (n = 11). The control group (n = 6) consisted of control vessels taken from a contralateral CCA that had no coil placement or surgical disruption. At harvest, unmodified coils in the sectioned CCA displayed minimal fibrosis around the coil or in the vessel wall when visualized through the surgical microscope. However, coils coated with collagen/rhVEGF showed clear evidence of thrombosis and fibrosis upon removal. Representative sections from each group stained with H & E are shown in Figure 2. Figure 2D shows massive intimal hyperplasia and substantial clot organization for coils coated with collagen/rhVEGF.
Histopathologic findings (stained with H&E) in the CCA segments for each group after 14 day coil implantation (original magnification ×20).
A, Contralateral control.
B, Coil.
C, Coil + collagen. D, Coil + collagen/rhVEGF.
Pathology Scoring
The results of the blinded pathology scoring are summarized in Table 1, with grading of intimal proliferation (0–3), percent of intimal occlusion (%), and grade of fibrosis (0–3). Intimal proliferation was graded on a scale of 0 for control CCA segment, 1 for mild proliferation, 2 for moderate proliferation, and 3 for severe proliferation. Intimal proliferation grades were significantly greater for coil + collagen/rhVEGF (2.8 ± 0.40) when compared with coil alone (1.4 ± 0.73; P < .001) and coil + collagen (1.6 ± 0.55; P < .001). Percent occlusion, determined using a control vessel as 0% occluded, showed a progressive increase in coil (18% ± 11%), coil + collagen (39% ± 20%), and coil + collagen/rhVEGF (87% ± 6%). Percent occlusion was significantly greater when comparing coil + collagen/rhVEGF with coil alone (P < .001) and coil + collagen (P < .001). Fibrosis of the arterial wall was graded on a scale of 0 for control CCA segment, 1 for mild fibrosis, 2 for moderate fibrosis, and 3 for severe fibrosis. Fibrosis was significantly greater in coil + collagen/rhVEGF (2.6 ± 0.50) when compared with coil alone (1.4 ± 0.55; P < .001), and marginally significant when compared with coil + collagen (2.0 ± 0.71; P = .06).
Mean pathology scores ± standard deviation by blinded observer for intimal proliferation, internal elastic lamina disruption, and fibrosis
Quantitation of Vessel Changes
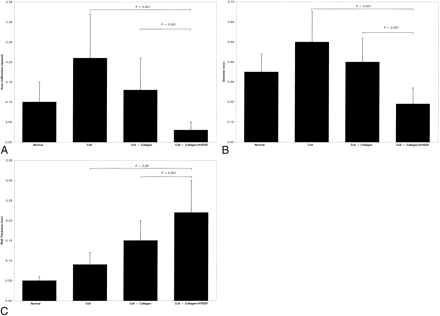
After 14 days of implantation, the mean area of the CCA lumen was 0.21 ± 0.11 mm2 with coil alone, 0.13 ± 0.08 mm2 with coil + collagen, and 0.03 ± 0.02 mm2 with coil + collagen/rhVEGF (Table 2 and Fig 3A). CCA lumen mean area was significantly smaller with coil + collagen/rhVEGF compared with coil alone (P < .001) and coil + collagen (P < .001). Mean lumen diameter of the CCA was 0.50 ± 0.15 mm with coil alone, 0.40 ± 0.12 mm with coil + collagen, and 0.19 ± 0.08 mm with coil + collagen/rhVEGF (Table 2 and Fig 3B). Mean diameter of CCA lumen with coil + collagen/rhVEGF was significantly smaller than with coil alone (P < .001) and coil + collagen (P < .001). Mean wall thickness of the CCA was 0.09 ± 0.03 mm with coil alone, 0.15 ± 0.05 mm with coil + collagen, and 0.22 ± 0.08 mm with coil + collagen/rhVEGF (Table 2 and Fig 3C). Mean wall thickness of CCA with coil + collagen/rhVEGF was significantly greater than coil alone (P < .001) and marginally significant when compared with coil + collagen/rhVEGF (P = .06).
Mean ± standard deviation of area, diameter, and wall thickness
Bar graphs comparing the results of the quantitative analysis.
A, Lumen area.
B, Lumen diameter.
C, Vessel wall thickness.
Factor VIII Immunostaining
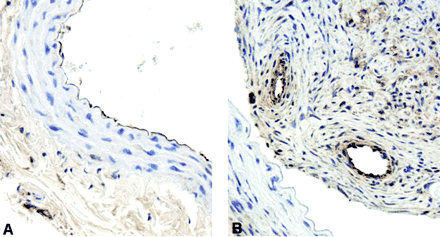
Histopathologic CCA sections from coil + collagen/rhVEGF were stained with Factor VIII to assess the presence of endothelial cells (Fig 4). In a control CCA, Factor VIII positivity can been seen along the endothelium (Fig 4A). However, the sections shown in Figure 2D showed positive staining for Factor VIII in areas of neovascularization in the fibrotic tissue mass (Fig 4B).
Immunohistochemical staining for Factor VIII.
A, Control CCA section showing positive staining along the endothelium (original magnification ×40).
B, CCA section from coil coated with collagen/rhVEGF with positive staining in areas of neovascularization (original magnification ×40).
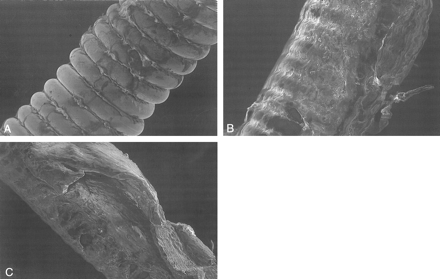
Scanning Electron Microscopy
Scanning electron microscopy was performed on coil segments from the different study groups (Fig 5). Unmodified coils showed minimal fibrotic accumulation on the surface (Fig 5A) and there was evidence of slightly more fibrosis on those coils modified with collagen (Fig 5B). Coils coated with collagen/rhVEGF showed more accumulation of cellular debris on the surface than did the coil alone or coil + collagen (Fig 5C).
SEMs of the surfaces of coil segments from different groups.
A, Unmodified coil (original magnification ×200).
B, Coil + collagen (original magnification ×200).
C, Coil + collagen/rhVEGF (original magnification ×150). Note the intense cellular reaction with coil + collagen/rhVEGF.
Discussion
The central goal of GDC therapy is to tightly pack an aneurysm with coils to promote thrombosis, fibrosis, and, ultimately, endothelialization across the aneurysm orifice. However, recent histopathologic follow-up data has begun to highlight some of the potential limitations of GDC therapy. Histopathologic characteristics of human aneurysms treated with GDCs have been assessed at autopsy (10–16). In general, aneurysms may appear completely occluded by angiography; however, histopathologic follow-up has shown that unorganized clot and small fluid spaces can exist between the coils in an otherwise treated aneurysm. Although microcoils packed within an aneurysm provide scaffolding that may benefit overall aneurysm stability and thrombus formation due to stasis, platinum is relatively biologically inert. Surface modification that increases biological activity could improve this technology and make GDC therapy more effective. Previous studies used in vitro and in vivo models to investigate surface modification of GDCs with extracellular matrix proteins (1, 3, 7, 17, 18), non-biodegradable polymers (5, 19), and ionizing radiation (6), and coating the coils with immortalized fibroblasts that secrete growth factors (3, 4).
Polyurethanes and polyesters have both been used to increase coil surface area and the fabric strands enhanced thrombogenicity (1, 5). Others have used type I collagen to coat the GDC surface and have shown a better cellular response, with more endothelium compared with unaltered coils (1, 18). Another modification is to fill the center of coils with collagen, which increases the number of fibroblasts on the coils compared with controls (2, 7). Muramaya et al (6) coated GDCs with albumin, fibronectin, and collagen and subjected them to high-energy neon ion implantation. Their results demonstrated that fibronectin-coated coils exposed to high-energy showed the greatest acute thrombogenicity. Kallmes et al (4) genetically modified fibroblasts with a basic fibroblast growth factor (bFGF) plasmid that was grown in culture medium with coils. These coils displayed greater fibroblast proliferation compared with unmodified coils when placed into the CCA.
VEGF is a potent angiogenic growth factor with direct and specific mitogenic effects on endothelial cells (20, 21). In local tissue responses, VEGF and bFGF have a synergistic effect on endothelial cell differentiation and angiogenesis. Studies have shown that expression of VEGF mRNA and subsequent angiogenesis increase in areas of hypoxic cells in vitro and near necrotic areas of glioblastoma cells in vivo (22–24). It has been shown that an intracoronary injection of recombinant VEGF (25), plasmid coding for VEGF (26), or adenovirus used for VEGF expression (27) promotes the development of collateral vessels within ischemic myocardial tissue. In addition, VEGF may promote collateral angiogenesis in patients with critical limb ischemia after intramuscular injection (28, 29), and in patients with ischemic myocardium after intracoronary (25) and intramyocardial injection (26, 27).
Although VEGF has played a major role in studies primarily focused on angiogenesis and formation of collaterals in myocardial and lower limb ischemia under conditions of high flow, we hypothesized that VEGF may promote a tissue response when coated onto GDCs under conditions of stasis and pressurization. Histopathologic follow-up after GDC therapy showed aneurysm fibrosis most pronounced at the periphery, suggesting that coil modifications to promote a reaction across the entire dome may be advantageous. By using recombinant growth factor, rhVEGF released from the coil could provide the biologic activity necessary to induce fibrosis across a vascular structure.
In this study, CCA segments treated with coil + collagen/rhVEGF showed a profound tissue response in the vessel lumen, and an accumulation onto the coil itself by SEM. Immunostaining with Factor VIII confirmed the presence of neovascularization within the fibrotic tissue mass, suggesting that rhVEGF coated onto GDCs may enhance tissue proliferation, especially in larger coiled aneurysms. While it is possible that rhVEGF has a thrombogenic potential distinct from its growth factor activity, it would be difficult to discern this thrombogenic potential above the massive response expected from the action of collagen alone on the small pool of blood available for clotting at the time of ligation in this no-flow model. The initial collagen to rhVEGF ratio on the coil is about 6:1 at the time of implantation.
Distinct from prior angiogenic work with VEGF delivery or expression after direct injection into muscle or arteries, the ligated artery does not provide an available microvasculature for cell recruitment, neocapillary formation, or red blood cell perfusion. This may explain why capillaries and red blood cells were not detected at the 2-week time point within the organizing fibrotic tissue within the ligated vessel lumen.
Evaluation of the coil surface after removal may be affected by the adhesion of material (fibrosis versus unorganized tissue) to the coil versus cohesion of this material to the vessel. It remains possible that “cleaner” coils can be pulled from fibrosed aneurysms, whereas material may be removed with the coil from unorganized tissue within the vessel lumen. We are not aware of any direct studies evaluating the adhesion strength of fibrosis, unorganized tissue, or blood clot to platinum microcoils. However, the massive organization of the lumenal tissue in vessels implanted with rhVEGF-coated coils is consistent with the observation of fibrotic (blood clot free) encrustation of the rhVEGF-coated coil surfaces as observed by SEM.
Augmented flow in the contralateral control is expected to cause some dilation and increase in lumen diameter, although changes in wall thickness may be small at the 2-week time point. We also used weight-matched animals with normal left and right carotids as donors for the control group and found no major differences from contralateral vessels obtained from rats with unilateral ligation. Although we used a simple in vivo model and not an experimentally created aneurysm, the addition of rhVEGF to the coil is not obvious on the basis of its use in myocardial ischemia. Our results suggest rhVEGF enhances fibrosis and induces new blood vessel formation that could improve fibrosis across the dome of larger aneurysms. In addition to recombinant expression, VEGF could also be expressed through plasmid or adenovirus.
Conclusion
We have shown in an in vivo model of arterial stasis and pressurization that rhVEGF added to the GDC surface provides an enhanced cellular response compared with unmodified coils and coils modified with collagen. Our results suggest that factors with enhanced biological activity that are added to GDCs may induce a better fibrotic reaction and improve occlusion rates of aneurysms, especially those that are large and/or with wide necks. The next step would be to use rhVEGF-modified coils in experimental jugular vein aneurysms to compare their efficacy to other GDC surface modifications.
Acknowledgments
The authors would like to thank Target Therapeutics, Inc. for their contribution of the Guglielmi detachable coils and Genentech, Inc. for their contribution of rhVEGF.
Footnotes
1 Presented orally at the Fourth Annual AANS/CNS Section Meeting on Cerebrovascular Surgery in Big Island, Hawaii on February 9–12, 2001.
↵2 Address reprint requests to John M. Abrahams, MD, Department of Neurosurgery, Silverstein 5, The Hospital of the University of Pennsylvania, 3400 Spruce Street, Philadelphia, PA 19104.
References
- Received November 17, 2000.
- Accepted after revision January 13, 2001.
- Copyright © American Society of Neuroradiology