Abstract
BACKGROUND AND PURPOSE: Most patients with tuberous sclerosis complex (TSC) do not receive prenatal diagnosis. Our aim was to describe MR imaging findings to determine the following: 1. Whether normal fetal MR imaging is more common in fetuses imaged at ≤24 weeks’ gestation compared with >24 weeks 2. The frequency of cardiac rhabdomyoma 3. The range of MR imaging phenotypes in fetal tuberous sclerosis complex.
MATERIALS AND METHODS: Our institutional fetal MR imaging data base was searched between January 1, 2011 and June 30, 2021, for cases of TSC confirmed either by genetic testing, postnatal imaging, postmortem examination, or composite prenatal imaging findings and family history. A MEDLINE search was performed on June 8, 2021.
RESULTS: Forty-seven published cases and 4 of our own cases were identified. Normal findings on fetal MR imaging were seen at a lower gestational age (mean, 24.7 [SD, 4.5 ] weeks) than abnormal findings on MR imaging (mean, 30.0 [SD, 5.3] weeks) (P = .008). Nine of 42 patients with abnormal MR imaging findings were ≤24 weeks’ gestation. Subependymal nodules were present in 26/45 cases (57.8%), and cortical/subcortical lesions, in 17/46 (37.0%). A foramen of Monro nodule was present in 15 cases; in 2/7 cases in which this was unilateral, it was the only abnormal cerebral finding. Cardiac rhabdomyoma was absent in 3/48 cases at the time of fetal MR imaging but was discovered later. Megalencephaly or hemimegalencephaly was observed in 3 cases.
CONCLUSIONS: Fetuses with abnormal cranial MR imaging findings were older than those with negative findings. Fetal hemimegalencephaly and megalencephaly should prompt fetal echocardiography. Cardiac rhabdomyoma was not always present at the time of fetal MR imaging.
ABBREVIATIONS:
- CR
- cardiac rhabdomyoma
- GA
- gestational age
- GE
- ganglionic eminence
- TSC
- tuberous sclerosis complex
- iuMR
- fetal MR imaging
- US
- ultrasound
Tuberous sclerosis complex (TSC) is an autosomal dominant genetic disease with an incidence of 1 in 6000. Its clinical manifestations are highly variable, but symptoms include seizures, mental retardation, skin lesions, and the formation of hamartomas in multiple organs, including the heart, brain, eye, and kidney.1 Autosomal dominant pathogenic variants in 1 of the 2 tumor-suppressor genes TSC1 and TSC2 are responsible for TSC,2 and more than half of cases are due to spontaneous (noninherited) mutations. Clinically unaffected parents with a child with TSC, however, have a 3% risk of recurrence in a subsequent offspring because of gonadal/germline mosaicism. It has been estimated that approximately 1% of individuals with TSC have germline3,4 and 15% have somatic mosaicism, likely accounting for the 20% of people meeting the diagnostic criteria for TSC who have no demonstrated genetic abnormality on peripheral blood testing.5 Although the presence of a pathogenic TSC1 or TSC2 variant is now a major diagnostic criterion for TSC, the difficulty in identifying the variant in a substantial proportion of patients with the condition underscores the importance of imaging, particularly in the prenatal and early postnatal period when cardiac and cerebral abnormalities are often the only stigmata of TSC. Additionally, prenatal and postconception genetic diagnoses of TSC in embryos and fetuses are not yet widely available within an appropriate timeframe in most settings that undertake pregnancy care. Judicious use of costly genetic testing is contingent on a prenatal imaging phenotype and family history. Moreover, the early use of mTOR inhibitors may ameliorate the clinical course and modify the clinical and imaging phenotype. Postnatal treatment of TSC with rapamycin and antiepileptic therapy with vigabatrin are well-established; however, there have been very recent case reports of successful in utero treatment with rapamycin of affected fetuses.6
While identification of one or multiple cardiac rhabdomyomas is the most common reason for TSC suspected on prenatal ultrasound (US) at or after the midtrimester, up to one-third of fetuses with cardiac tumors do not have TSC.7 In the absence of genetic confirmation, a diagnosis of TSC requires the presence of at least 2 major or 1 major and 2 minor criteria; 2 major criteria are subependymal nodules and cortical tubers.5 However, fetal cranial manifestations of TSC are not confined to these 2 abnormalities despite their acknowledged importance as primary diagnostic criteria.8
Improved understanding of the range of abnormal fetal brain phenotypes in TSC and their variation with gestational age may help identify potentially affected fetuses earlier; triage pregnancies appropriately for more efficient and cost-effective genetic testing and prenatal surveillance with cardiac sonography when cardiac tumors are not present; and initiate intrauterine rapamycin treatment earlier when this treatment becomes more mainstream.
Aims
To report on the prenatal MR imaging findings in 4 fetuses from a single institution with TSC and review the literature on cranial abnormalities on MR imaging in fetuses with TSC to more fully describe and understand the prenatal TSC phenotype and determine whether:
Normal fetal MR imaging (iuMR) is reported more commonly in fetuses ≤24 weeks’ gestation compared with >24 weeks
Any cases of TSC diagnosed by iuMR had neither cardiac rhabdomyoma nor known family history at the time of iuMR.
MATERIALS AND METHODS
A data base review was conducted at our own institution to identify fetuses with a confirmed diagnosis of TSC who had iuMR during the past 10 years. A waiver of institutional review board approval for this activity and for use of these de-identified patient data for research and teaching purposes was obtained. In addition, a literature search of MEDLINE was conducted on June 8, 2021, using the following search terms: (Fetal or Fetus) and (MR imaging or Magnetic Resonance) and (tuberous sclerosis). Full-text articles were accessed for all retrieved citations, and inclusion and exclusion criteria were applied. For studies to be included, a description of iuMR findings for fetuses with TSC had to be provided and/or illustrated. Letters, case reports, short communications, and case series and other research publications were considered if they were published in English and if the diagnosis of TSC were ascertained through composite prenatal imaging findings/family history consistent with the diagnosis and/or genomic confirmation and/or postmortem/postnatal diagnosis of TSC. Reference lists of retrieved studies were scanned for potentially eligible additional studies that were not retrieved in the original search. Studies were excluded if details of the iuMR findings in individual TSC cases could not be extracted from provided images or if
The imaging findings in individual cases were not described (ie, only aggregated data were reported) or
The gestational age of the fetus at the time of iuMR was not provided.
In addition to the literature search, we conducted an audit of our institutional iuMR data base between January 1, 2011, and June 30, 2021, and applied the same inclusion/exclusion criteria as used for the MEDLINE search.
Data Extraction
When a case was reported as having abnormal findings, the reported and/or depicted cranial imaging findings were categorized as present, absent, or not stated. “Not stated” was when the particular domain or feature was not specifically mentioned as being present or absent in the report of the case and the provided images did not permit confident confirmation of presence or absence of the finding.
The range of tabulated cranial abnormalities was determined by findings in previous case reports and on the basis of our clinical experience in our own cases. When cranial findings on iuMR for a case were reported as being normal, all domains were scored as absent. These domains were the following: foramen of Monro mass/ganglionic eminence (GE) enlargement (unilateral or bilateral), subependymal nodules, cortical or subcortical nodules, dominant hemispheric mass, hemimegalencephaly, megalencephaly, white matter signal abnormalities/transmantle sign, ventriculomegaly, and cerebellar abnormality. In addition, fetal gestational age at iuMR, the presence of cardiac tumors or renal cysts, and a positive family history were tabulated.
RESULTS
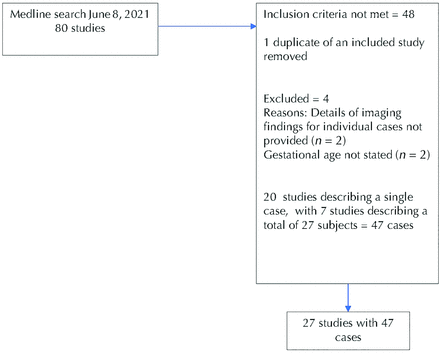
Seventy-nine primary studies were retrieved as a result of the MEDLINE search (Fig 1). An additional 2 studies meeting the inclusion criteria were identified from scanning the reference lists of the retrieved studies; a literature review and case report9 and a letter reporting a single case10 were identified in this way.
Four additional cases were identified following an audit of our institutional iuMR data base (Figs 2–4), 2 of which were included in a prior publication on imaging of fetal GE abnormalities,8 with a total of 51 cases (Fig 1). Details of included and excluded studies with full citations are provided in the Online Supplemental Data.
Case ascertainment process.
Thirty-two to 40 weeks’ gestation. 3T, single-shot T2-weighted EPI shows the foramen of Monro dominant nodule/enlarged GE (A and B) and cortical tuber (C).
Twenty-five weeks’ gestation. A 1.5T, single-shot T2-weighted EPI (A) shows the right foramen of Monro dominant nodule/enlarged GE and the normal GE on the left. T2*-weighted image (B) demonstrates no blood products in the mass.
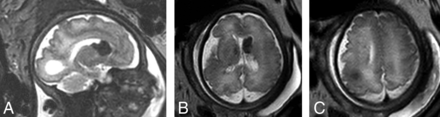
Twenty-two weeks’ gestation. A 1.5T single-shot T2-weighted EPI demonstrates hemimegalencephaly and a dominant ipsilateral mass representing a possible coexistent subependymal giant cell astrocytoma. This was initially interpreted on screening US as a parenchymal hemorrhage. After the iuMR, dedicated fetal echocardiography the following day identified a single small rhabdomyoma.
Twenty studies each reported the findings of a single fetus. Seven of the included studies reported imaging findings in >1 case that met the inclusion criteria, accounting for a further 27 cases: Ulm et al11 (n = 6), Zhou et al12 (n = 5), Mühler et al13 (n = 5), Jurkiewicz et al14 (n= 5), Levine et al15 (n = 2), Prabowo16 (n = 2), and Sonigo et al17 (n = 2).
The mean gestational age (GA) for the 51 fetuses was 29.1 weeks, and the mean GA for the 4/51 new cases from our institution was 27.3 weeks. For the 9 fetuses with normal MR imaging findings, the mean GA was 24.7 (SD, 4.5) weeks, whereas for those with abnormal MR imaging findings, the mean GA was 30.0 (SD , 5.3) weeks (P = .008). Eleven fetuses were reported as having a family member affected with TSC. Of 20 cases in which the field strength for the iuMR examination was reported, 2 studies were performed at 3T and the other 18 at 1.5T.
Imaging Findings
Details of the imaging findings at iuMR for individual included cases are provided in the Online Supplemental Data. These are summarized in Tables 1 and 2.
Cranial MR abnormalities in fetuses with TSCa
Cranial abnormalities in fetuses with TSC: combined data from literature review and local institutional casesa
Subependymal nodules were present in 26 of 45 cases (57.8%), and cortical/subcortical lesions, in 17 of 46 (37.0%). In 8 of 19 (42.1%) cases demonstrating ventriculomegaly, only 1 had this as an isolated cerebral finding in a fetus with a GA of 26 weeks18 with cortical tubers evident on postnatal MR imaging despite sirolimus administration from 28 weeks’ gestation.
A foramen of Monro nodule was present in 15 cases; in 8 of these, it was bilateral, and in 7, unilateral. Of the 7 unilateral cases, in 2 of 7, it was the only reported abnormal cerebral finding, and in a third case, one of our own, was accompanied only by mild ventriculomegaly. However, all 3 cases were referred for iuMR due to cardiac tumor.
Megalencephaly, hemimegalencephaly, and cerebellar abnormality were very uncommon, being observed in 2/18, 1/18, and 1/18 fetuses, respectively. The fetus with cerebellar abnormality had other characteristic features of TSC, including subependymal nodules and cortical mass lesions. The cases with megalencephaly or hemimegalencephaly had ≥1 cardiac mass, though in 1 of the 2 cases of hemimegalencephaly previously reported from our institution as part of another study,8 the single cardiac ventricular rhabdomyoma became evident only when fetal echocardiography was repeated the day following the MR imaging, which had been performed due to a suspicion on prenatal US of a cerebral hemispheric mass lesion or hemorrhage.
The phenotypic manifestations of TSC in the group of fetuses of ≤24 weeks’ gestation (n = 9) are of particular interest. A dominant hemispheric mass was present in 5 (55%), and this was associated with either subependymal nodules (n = 1) or unilateral GE enlargement. In only 2 of the 9 cases were subependymal nodules alone or unilateral GE enlargement alone identified, and in the 2 remaining cases, a combination of subependymal nodules, cortical/subcortical nodules, and a foramen of Monro mass lesion was present.
Of 9 patients with normal fetal cranial MR imaging findings, all of whom had ≥1 cardiac rhabdomyoma (CR), repeat MR imaging, performed between 3 and 12 weeks later in 3 cases, demonstrated cerebral abnormalities consistent with TSC; all of these repeat studies occurred during the third trimester of pregnancy at 26–34 weeks’ gestation.
Furthermore, only 3 of the 48 (6.7%) fetuses with description of the presence or absence of a cardiac mass had no sonographic evidence of a cardiac mass at the time of iuMR. In 2 of these, including the case of hemimegalencephaly from our institution, the fetus’s father was known to have TSC. In all 3 cases, the CR was demonstrated on a second US performed between 1 day and 4 weeks after the iuMR at a gestational age of between 22 and 34 weeks.19,20
The presence or absence of renal cysts was reported in only 3 cases, apart from the 4 from our institution, none of whom had cysts identified on US. Of these 3, only 1 had cysts and also had evidence of CR.21
DISCUSSION
The current review, including 4 cases from our own institution, establishes that a cardiac tumor was present at the time of iuMR in all except 3 of 51 cases of proved TSC, and in 2 of these 3 cases, the fetus’s father had known TSC. This finding highlights the importance of a careful search for CR in fetuses with cranial abnormalities suggestive of TSC because CRs substantially increase the pretest probability of TSC and their absence should raise questions about the diagnosis, especially in a fetus with no family history.
Similarly, when a unilateral foramen of Monro nodule or mass, essentially synonymous with unilateral GE enlargement, is seen as an isolated abnormality, or with only ventriculomegaly, TSC should be considered if a CR is present. Unilateral germinal matrix hemorrhage can mimic this appearance; consequently, routine performance of susceptibility-weighted imaging and fetal echocardiography can help avoid overdiagnosis of TSC, with resulting inappropriate prognostic and recurrence counseling in this situation.
The incidence of CR in patients with TSC is 50%–80%,22 with a recent study suggesting that only 22% of a cohort of patients with TSC had CR diagnosed prenatally. Moreover, CRs can appear late in pregnancy, most often after the midtrimester screening examination is performed.23 Thus, it is possible that a substantial number of fetuses with TSC will not have this diagnosis suggested prenatally by the presence of CR. While intracranial manifestations of TSC on iuMR might, therefore, be the first indication of the disorder due to iuMR being more sensitive and specific than prenatal US for intracranial pathology,24 this was not borne out by our literature review. This may, however, reflect selection bias in that reported cases of TSC on iuMR have been referred for MR imaging due to the identification of CR either at the midtrimester screening examination or in the third trimester as an “incidental” finding when growth, placental position, or other surveillance has occurred.
We have identified that among fetuses with proved TSC who have had iuMR, the likelihood of abnormal cranial findings varies significantly with GA. Fetuses who have abnormal findings were, on average, at 30 weeks’ gestation compared with 25 weeks for fetuses with no abnormality. Interestingly, most fetuses with TSC, when the diagnosis was made at 24 weeks or earlier in gestation, demonstrated a dominant hemispheric mass, whereas only 1 fetus (11% of this early-gestation cohort) had subependymal nodules as their sole imaging manifestation. This finding makes intuitive sense because more severe “masslike” lesions would be more likely to be detected at the midtrimester screening US examination, typically performed between 19 and 21 weeks’ gestation, and would manifest as a midline shift. Such masslike presentations at screening US can be misinterpreted as hemorrhage and underscore the importance of careful tertiary echocardiography and T2*-weighted iuMR for further fetal evaluation.
While association of cranial abnormalities with increasing gestation might suggest that it is better to wait to perform iuMR, 9/42 (23%) fetuses with abnormal cranial MR imaging findings were 19–24 weeks’ gestation when MR imaging was performed. This timing is potentially important in jurisdictions where GA-related limitations exist on pregnancy termination. It may also be salient to the commencement of intrauterine therapy with rapamycin. When genomic testing is either unavailable or not turned around quickly enough to be clinically useful regarding counseling and therapeutic decision making, earlier diagnosis may also be important. However, “early” (<25 gestational weeks) MR imaging may be associated with a higher rate of negative findings; thus, it may be a more costly strategy if a second iuMR is performed later in pregnancy after initial negative examination.
Improvements in ultrasound equipment during the past decade and in particular the evolution of the tertiary neurosonogram using a transvaginal approach and, when possible, the sonographic window of the anterior fontanelle have dramatically improved the quality of fetal brain imaging with US. In addition, the spatial resolution of US is superior to that of MR imaging, theoretically making subependymal nodule detection easier with US than with MR imaging. However, tertiary neurosonography remains highly operator-, patient-, and fetal position–dependent. Whether such optimized US can detect subependymal nodules in practice in fetuses at a high demographic risk of TSC when these are not demonstrable with iuMR is a question outside of the scope of the current review.
Finally, by aggregating data from multiple case reports and case series, the current study highlights some less common presentations of TSC, in particular hemimegalencephaly and megalencephaly. Because other genetic disorders, in particular other mTOR pathogenic variants, such as PIK3CA-related overgrowth spectrum disorders, are the underlying pathogenic variants in most cases of megalencephaly or hemimegalencephaly and these are almost invariably de novo mutations or, much less often, the result of germline mosaicism, distinguishing TSC-related hemimegalencephaly/megalencephaly from these more common non-TSC causes is very important. It is theorized that because activation of TSC1 and TSC2 is driven by upstream signals of the PIK3/AKT pathway, the loss-of-function pathogenic variants of TSC1 or TSC2 that result in clinical TSC could induce an imbalance in PIK3/AKT pathway functioning, producing cerebral hemispheric overgrowth.10 Hence, careful examination and re-examination of the fetal heart with tertiary fetal echocardiography are recommended in the presence of hemimegalencephaly/megalencephaly in the fetus. These will help direct genomic testing and prevent inappropriate advice regarding the utility of such testing or the likelihood of recurrence in subsequent pregnancies.
Our study has limitations, mainly due to potential selection bias in published cases driven by a prenatal US diagnosis of CR being the trigger to refer a patient for iuMR. Because it is recognized that CR may not be detectable with US until the third trimester of pregnancy and frequently not until the postnatal period, the cases included in this review are likely to provide a biased impression regarding the following:
The likelihood of TSC being the cause for fetal cerebral abnormalities when there is no CR or family history (because the pregnancy may be terminated without a genetic diagnosis or postmortem examination)
How early in gestation the cerebral lesions of TSC can be diagnosed with MR imaging in fetuses with high pretest risk
How often iuMR findings are negative for cerebral lesions in fetuses with high pretest risk of TSC (because of family history of the condition) and at what gestational age, because cases with negative findings are less likely to be published.
A prospective cohort study of serial MR imaging in fetuses who are the offspring or siblings of individuals with TSC would provide a more accurate estimate of these important issues to help inform clinical practice. However, pre-implantation genetic diagnosis and/or gene panel testing for pathogenic TSC1 and TSC2 variants during early pregnancy may become more feasible, economical, and acceptable to patients than second trimester iuMR screening when the fetus has a high risk of the condition due to an affected sibling or parent. In addition, only a small minority of fetuses reported in this study were imaged using a 3T scanner; the reduced signal-to-noise and thus the resolution of iuMR at 1.5T may reduce the sensitivity for cerebral abnormalities in fetuses with TSC.
A further limitation of the current study is due to variable terminology used to describe iuMR imaging findings and, in particular, the general lack of routine reporting of cranial biometry. Specifically, when iuMR was said have normal findings but it was unclear whether fetal brain biometry had actually been performed, it is possible that megalencephaly with a “normal”-appearing brain may have been underrecognized. Moreover, the range of terms used to describe a mass, nodule, or swelling in the region of the foramen of Monro or GE of a fetus on MR imaging may have made the distinction among a foramen of Monro “mass,” a small hemispheric mass, and subependymal nodule in the region of the foramen of Monro an artificial one. Hence, we may have misclassified abnormalities in this region when relying on descriptive terms used in the report, mainly because their imaging appearances can overlap.
CONCLUSIONS
The current study highlights the ability of iuMR to provide the diagnosis of TSC in fetuses ≤24 weeks’ gestation and underscores the importance of considering the possibility of TSC when fetal megalencephaly or hemimegalencephaly or a masslike cerebral lesion are observed; these findings should trigger tertiary fetal echocardiography which, if normal, may need to be repeated later in pregnancy. Whole-exome sequencing and specific TSC genetic testing will be increasingly used to provide an earlier prenatal diagnosis when family history, the presence of CR, or iuMR cerebral phenotype suggests potential TSC.
Acknowledgments
The authors would like to thank the families who permitted their fetuses’ imaging data to be provided in this publication.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received October 19, 2021.
- Accepted after revision January 10, 2022.
- © 2022 by American Journal of Neuroradiology