Abstract
BACKGROUND AND PURPOSE: Alveolar soft-part sarcoma (ASPS) of the head and neck is an extremely rare malignancy. Although the clinical and imaging features of this tumor have been reported, a periodic review of unusual tumors is useful. The purpose of this study was to describe the clinical and imaging features of ASPS of the head and neck.
METHODS: Between January 1990 and May 2004 at our institution, five head and neck ASPS were diagnosed in five patients (two male and three female patients; age range, 4–22 years). Clinical and imaging findings were reviewed retrospectively. Imaging studies consisted of contrast material-enhanced CT (in four patients), MR imaging (in four patients), and digital subtraction angiography (in two patients).
RESULTS: The locations of the tumor were tongue in two cases, larynx in one case, buccal space in one case, and paravertebral space in one case. This tumor appeared as a large lobulating-contoured mass with high signal intensity and flow voids on T2-weighted images and showed strong enhancement on contrast-enhanced CT and MR images. Preoperative angiography showed high vascularity. Wide surgical excisions were performed in four cases. Mean follow-up periods were 16 months (range, 6–30 months), and no recurrence was noted except for the laryngeal case.
CONCLUSION: ASPS should be included in the differential diagnosis of head and neck tumor when a slow-growing, large mass with high signal intensity and flow voids on T2-weighted images and strong enhancement on contrast-enhanced CT or MR image is seen, particularly in young female patients.
Alveolar soft-part sarcoma (ASPS) is a rarely encountered type of soft-tissue sarcoma that primarily affects teenagers and young adults. ASPS is most commonly seen in the upper and lower extremities and is rarely seen in the head and neck region (1). The tumor is highly vascular and often manifests with metastasis, particularly in cases in the extremities. The lung is the most common site of metastasis (2). This neoplasm mimics benign vascular neoplasm or malformations (3). However, certain distinctive clinical and radiologic features suggest the correct diagnosis (4). We report five cases of ASPS of the head and neck and emphasize clinical and imaging features of this tumor.
Methods
The radiologic images obtained at two institutions between January 1990 and May 2004 in five patients with ASPS of the head and neck (two male and three female patients; age range, 4–22 years; mean age, 14 years) were retrospectively reviewed. Four of these five patients presented with painless swelling at the tongue (cases 1 and 2), cheek (case 4), and neck (case 5). A patient with a laryngeal lesion (case 3) presented with hoarseness but without pain. Wide surgical excisions were performed in four cases. In the laryngeal case, as the patient’s parents refused surgical procedures including total laryngectomy and tracheostomy, the patient was treated with radiation and chemotherapy. The clinical findings in the five patients with ASPS are summarized in the Table.
Clinical findings in five patients with a diagnosis of ASPS in the head and neck
The imaging characteristics were evaluated by two head and neck radiologists before surgery or pathologic confirmation, who were unaware of the patient’s information, with consensus on imaging findings. The imaging studies reviewed consisted of MR images, including T1-, T2-, and gadolinium-enhanced T1-weighted images in the axial, coronal, and sagittal planes (cases 1, 2, 4, and 5), contrast-enhanced CT scans (cases 2, 3, 4, 5), and digital subtraction angiography (DSA, cases 4 and 5). The two patients who underwent DSA were treated with tumor embolization. The radiologic features were reviewed for the following characteristics: lesion size (the greatest diameter) and location, lesion margin and shape, signal intensity and attenuation, degree of contrast enhancement, internal architecture, the presence or absence of local tumor invasion, site of metastasis on CT and MR images, and vascularity on DSA images. We compared imaging features seen on MR images, CT scans, and DSA images with the pathologic specimen.
Results
Imaging Features
The tumor size averaged 7.2 cm in greatest dimension (range, 3.5–9 cm). The locations of the tumors were the tongue in two cases, the larynx in one case, the buccal space in one case, and the paravertebral space in one case. The shapes and margins of the tumors were round or ovoid, and they had a lobulated contour (Fig 1A). Contrast-enhanced CT scans showed strong tumor enhancement in all cases studied with CT (Figs 2A and 3A). T1-weighted MR images showed iso- to slightly hyperintensity of the tumor compared with that of normal neck muscles (Figs 1A and 2B). T2-weighted images showed the tumor to have high signal intensity and vascular signal voids (Fig 2C). Gadolinium-enhanced MR images showed strong enhancement and intratumoral signal voids (Fig 1B and 4). Local tumor invasion into surrounding structures was not seen in any study patient. Lung metastasis was seen in one patient, with the laryngeal lesion. Preoperative tumor embolizations were performed in two cases (cases 4 and 5) and preprocedural angiography showed prominent tumor staining (Fig 5B).
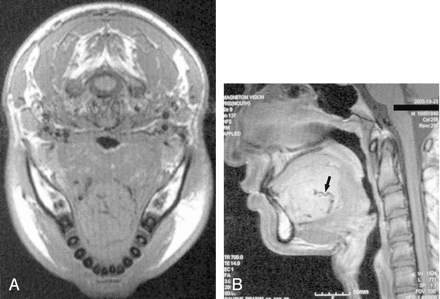
MR images of ASPS in a 16-year-old boy with tongue mass (case 1).
A, Axial T1-weighted MR image shows a large tongue mass with isointensity compared with neck muscles.
B, Sagittal contrast-enhanced T1-weighted MR image shows a large tongue mass with strong enhancement and signal voids in it (arrow).
CT and MR images of ASPS in a 4-year-old girl with tongue mass (case 2).
A, Contrast-enhanced CT scan shows a large tongue mass with strong homogeneous enhancement in the left side of the tongue.
B, Coronal T1-weighted MR image shows a large tumor with slightly high signal intensity in the tongue.
C, Coronal T2-weighted MR image shows a high-signal-intensity tumor with vascular signal voids (arrow) in the tongue.
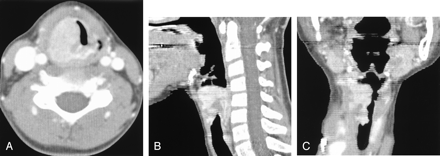
CT scans of ASPS in a 10-year-old girl with laryngeal mass (case 3).
A, Contrast-enhanced CT scan shows a lobulating-contoured mass with strong enhancement in the right true vocal cord.
B and C, Sagittal (B) and Coronal (C) reconstruction images show that the tumor extends from the right pyriform sinus to the upper trachea level.
MR image of ASPS in a 16-year-old girl with mass in the left paravertebral space (case 5). Axial contrast-enhanced T1-weighted MR image shows a large paravertebral space mass with strong enhancement and signal voids in it.
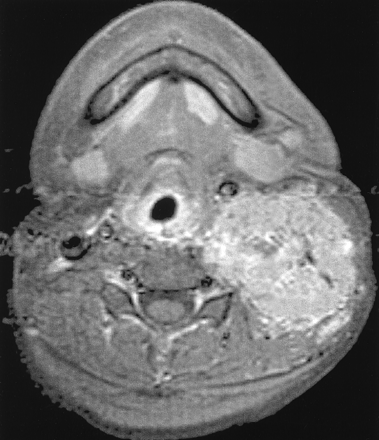
MR and DSA images for preoperative tumor embolization of ASPS in a 22-year-old man with buccal space mass (case 4).
A, Axial contrast-enhanced T1-weighted MR image shows a high-signal-intensity tumor with vascular signal voids in the buccal space.
B, Lateral projection of right external carotid angiogram shows prominent tumor staining supplied from branches of the internal maxillary artery.
Pathologic Findings
On histopathologic examination, the tumor was encapsulated. Microscopically, the tumor cells had relatively uniform nuclei, abundant eosinophilic cytoplasm, and prominent nucleoli and were arranged in an alveolar pattern separated by thin fibrovascular stroma. On immunohistochemical stainings, the tumor cells were negative for cytokeratin and contained periodic-acid-Schiff (PAS)-positive and diastase-resistant rhomboid or rod-shaped crystals in the cytoplasm. These pathologic features were consistent with ASPS.
Follow-up
Average follow-up period was 16 months (range, 6–30 months). During the follow-up periods, CT scans were obtained in three patients (cases 1–3), MR images in two patients (cases 4 and 5), and endoscopic biopsy in one patient (case 3). No evidence of local tumor recurrence was seen in four cases treated with wide surgical excision. However, in the patient with laryngeal ASPS who was treated with radiation and chemotherapy, an increase in primary tumor size was seen on follow-up neck CT scans 6 weeks after chemotherapy. This patient underwent a metastatic work-up with chest CT 7 weeks after initial presentation; the CT scan showed multiple metastatic nodules in both lungs.
Discussion
ASPS was first described as a disease entity in 1952 by Christopherson et al (5). The histologic features of the tumor are distinctive. Cytoplasmic crystals containing diastase-resistant, PAS-positive material are described as the most characteristic component of the tumor cells. The histologic origin of the tumor remains uncertain. On the basis of electron microscopic findings, myogenic or neural crest origin has been reported (6). The tumor’s common occurrence in skeletal muscle and desmin reactivity support a myogenic origin. Although recent studies have been unable to confirm myoD1 expression or myogenin reactivity (7), this may be attributed to its derivation from a myoid precursor with lack of differentiation (3). In more recent years, histochemical markers have tended to support the myogenic theory, with more chromosomal structural abnormalities found in a number of ASPS, including involvement of 17q,25 (8). Saito et al (9) reported a possible association between tumor progression and genetic alteration in ASPS. In that study, p53 gene mutations occurred in three (27.3%) of 11 cases of ASPS, similar to findings reported in other types of sarcomas (10). Furthermore, the E-cadherin gene mutation was detected in one case (9.1%) and the APC gene mutation was observed in one (9.1%) of 11 cases (9).
ASPS is an extremely rare malignant tumor composing less than 1% of soft-tissue sarcomas and accounting for less than 0.1% of sarcomas of the head and neck. The average age onset of the tumor is the second decade, with a female preponderance. This tumor is most commonly found in the lower extremities (44%) (11). Twenty-seven percent of the cases of ASPS involve the head and neck, with 41% of these lesions involving the orbit and 25% occurring in the tongue, which happens to be the most common site in infants and children (3, 12). The clinical course of the disease is slow; however, a significant proportion of patients experience relentless progression of the disease, with a high proportion of patients developing distant metastases during an extended follow-up (13). Metastasis is almost always by the hematogenous route, and the lungs are the most frequent site of metastasis. Lung metastases are seen in 42–65% of patients at presentation (14). Lymphatic metastasis is seen in only 10% of reported cases (3, 6). An ASPS of the head and neck often manifests with functional impairment related to the primary lesion, such as difficulty speaking in the case of a tongue lesion or hoarseness due to a laryngeal lesion, as was seen in our cases. Therefore, these lesions manifest at a lower stage than do extremity lesions, which frequently manifest with symptoms related to metastasis (6, 14).
Treatment has been surgical in most of the reported ASPS cases. To minimize local tumor recurrence, wide surgical excision of the primary tumor is usually performed without a neck dissection, unless palpable nodes are present. It seems that radical surgery fails to prolong survival. The response to radiation therapy or chemotherapy is poor. Lieberman et al proposed adequate surgical excision of the primary lesion as a treatment and preferred the same surgical approach for metastatic lesions (13). Prognosis is poor because of the high frequency of metastatic disease. Although prolonged survival is possible even in patients with metastasis, the long-term disease-specific mortality rate is high. In a large series, the median survival time was 3 years if metastatic disease was present at diagnosis and 11 years without metastatic disease at presentation. Local failures have been reported in up to 20% of patients with ASPS. Lieberman et al (13) found a 5-year survival rate of 59%, a 10-year survival rate of 38%, and a 20-year survival rate of 15%. Sherman et al (15) documented excellent local control in six adults given adjuvant or preoperative radiation therapy. However, the use of such regimens in children has been recommended only for those patients in whom surgical margins are inadequate or there is microscopic residual tumor, and in palliative therapy in patients with metastatic disease at selective sites (16).
Regarding the imaging features, it has been noted that this tumor shows high signal intensity on T1- and T2-weighted images compared with surrounding structures and has strong enhancement on contrast-enhanced CT and MR images; frequently, vascular signal voids and central necrosis are seen (3). These reliable radiologic findings are well correlated with the high vascularity of this tumor on pathologic specimens. In most of our cases, the tumor showed high signal intensity on T2-weighted images compared with a surrounding structure, was well enhanced on contrast-enhanced CT and MR images, and had vascular signal voids. However, in our cases, the tumor did not have a definite hyperintensity on T1-weighted images or central necrosis on T2-weighted or contrast-enhanced images. A laryngeal ASPS has very rarely been reported. De Sautel et al (11) reported a case of laryngeal ASPS in which CT scanning of the neck showed a large paraglottic tumor extending from the left aryepiglottic fold to the left true vocal cord. In our case of a laryngeal ASPS, the tumor also showed longitudinal extension from the right pyriform sinus to the upper trachea, as De Sautel et al reported, and a lobulating-contoured mass with strong enhancement on contrast-enhanced CT scans as seen in our other cases. Several imaging features including large tumor size, high signal intensity on T2-weighted images, flow voids in the tumor, and strong enhancement facilitate making the correct diagnosis of this tumor. Aiken and Stone (3) reported that this neoplasm may mimic hemangioma or vascular malformation, but careful evaluation of its unique imaging features on CT and MR images leads to the correct diagnosis. Our differential diagnoses included hemangioma, vascular malformation, squamous cell carcinoma in cases 1 and 2; hemangioma, vascular malformation, and paraganglioma in case 3; hemangioma, rhabdomyosarcoma, and minor salivary gland tumor in cases 4; and rhabdomyosarcoma, paraganglioma, and schwanoma in case 5. As seen in previous reports (3) and in our cases, the signal intensities of ASPS were not as intense on T1- and T2-weighted images as those seen with benign vascular tumors including hemangioma. Unlike ASPS seen in our cases and previous reports, squamous cell carcinoma of the tongue typically does not have flow voids, intense enhancement, and high signal intensity on T2-weighted images (3). Most rhabdomyosarcomas are generally found in the orbit or oral cavity or at the base of the skull (2).
Conclusion
The clinical and imaging features of ASPS of the head and neck have been rarely reported. Several clinical and imaging features, including young female, slowly growing tumor, high signal intensity on T2-weighted images, strong enhancement, and flow voids, reviewed in our report could be helpful for including this rare tumor in the differential diagnosis of head and neck tumors.
References
- Received August 11, 2004.
- Accepted after revision October 23, 2004.
- Copyright © American Society of Neuroradiology