Abstract
BACKGROUND: Several case series and prospective cohorts have reported the use of stent retrievers (SR) and specifically designed expanding stents (ES) to perform in situ mechanical stent angioplasty to treat cerebral vasospasm in subarachnoid vasospasm.
PURPOSE: The aim of this study was to review and conduct a meta-analysis to evaluate the safety and efficacy of this novel technique.
DATA SOURCES: A systematic review and meta-analysis was conducted according to established protocols. Searches were conducted in PubMed, Scopus, Web of Science, and EMBASE databases up to June 2024, including variations of “stent,” “expanding device,” “vasospasm,” “subarachnoid hemorrhage.” Original studies reporting treatment outcomes for vasospasm by using SR/ES in more than 5 patients were included.
STUDY SELECTION: Pooled data from 8 studies, comprising 156 patients and 428 targeted vessels treated with stent angioplasty for vasospasm were analyzed.
DATA ANALYSIS: We evaluated rates of angiographic success, complications, recurrence, and neurologic improvement. Meta-analysis was performed by using a random-effects model.
DATA SYNTHESIS: The angiographic success rate was 81.8% (95% CI: 70.6–89.3). Subgroup analysis showed a success rate of 86.5% (95% CI: 62.6–96.1) with ES and 80.5% (95%CI: 62.6–93.1) with SR. Overall complication rate was 1.1% (95% CI: 0.0–3.6), due to clot formation or hemorrhage. Recurrence of vasospasm was noted in 12.8% (95% CI: 5.2–28.1) while neurologic improvement was seen in 65.9% (95% CI: 51.1–78.1) of the cases. Finally, it should be noted that all included studies used stent angioplasty in combination with intra-arterial vasodilators.
LIMITATIONS: Our meta-analysis is limited by selection and reporting biases, as well as high heterogeneity. Moreover, the overall low quality of available evidence is the main limitation of our results.
CONCLUSIONS: Combination of stent angioplasty and intra-arterial vasodilators was found to have high rates of angiographic success and low incidences of adverse events. Randomized controlled trials are needed to confirm their efficacy and safety compared with medical and balloon angioplasty treatments.
ABBREVIATIONS:
- aSAH
- aneurysmal SAH
- ES
- expanding stent(s)
- SR
- stent retriever(s)
SUMMARY
PREVIOUS LITERATURE:
In situ mechanical stent angioplasty to treat cerebral vasospasm in subarachnoid vasospasm have been reposted recently in multiple case-series.
KEY FINDINGS:
Combination of stent angioplasty and intra-arterial vasodilators was found to have high rates of angiographic success and low incidences of adverse events.
KNOWLEDGE ADVANCEMENT:
Randomized controlled trials are needed to confirm their efficacy and safety compared with medical and balloon angioplasty treatments.
Cerebral vasospasm is a severe and life-threatening complication that can occur after aneurysmal SAH (aSAH). Angiographic vasospasm can be detected in up to 70% of patients with aSAH; however, it does not always correlate with the patient’s symptoms.1 Early diagnosis and treatment are essential to avoid delayed cerebral ischemia, which is the leading cause of morbidity and mortality in patients hospitalized for aSAH.2 In addition to medical management with oral nimodipine and euvolemia maintenance, there is no consensus on more aggressive treatments, including angioplasty or induced hypertension, and despite hypertension being commonly used there is no randomized controlled trial–level evidence of its benefit and some evidence to suggest it results in harm.3,4
Angioplasty can be performed mechanically with balloons, typically up to the end of the first segment of cerebral arteries,5⇓-7 or it can be performed utilizing intra-arterial vasodilator infusion therapy (chemical angioplasty).8 Although balloon angioplasty for vasospasm is broadly used in many centers, its benefit is still disputed.9 The main limitation is due to the risk of hemorrhagic and/or thromboembolic complications.10
In response to the need for durable and safe angioplasty technique to dilate cerebral vessels without increasing the risk of vessel rupture, Bhogal et al11,12 introduced the concept of treating vasospasm with stent retrievers (SR)—normally used for mechanical thrombectomy—in 2017, and expanding stents (ES)—specifically developed for this indication—in 2021. Since then, several other studies have reported success in by using stent angioplasty to resolve vasospasm.13,14 This systematic review and meta-analysis aims to provide a comprehensive understanding of the efficacy, safety, and potential complications associated with stent angioplasty by using SR and ES in treating cerebral vasospasms.
MATERIALS AND METHODS
Study Design and Search Strategy
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 guidelines. A systematic literature review was conducted on June 5, 2024, by using PubMed, EMBASE, Scopus, and Web of Science databases. Combinations of keywords and Medical Subject Headings (MeSH) terms were used to find the articles related to SR use in the event of SAH-related cerebral vasospasms. Keywords and MeSH terms included the following: “stent,” “stent retriever,” “Comaneci,” “cascade,” “expanding device,” “vasospasm,” “subarachnoid hemorrhage,” “SAH-related,” and “SAH.”
Screening Process
Studies in compliance with our predetermined Population, Intervention, Comparison, and Outcome (PICO) were included. The population of interest was patients experiencing cerebral vasospasms resistant to pharmaceutical treatments. The intervention was any type of SR/ES, and the comparison was not specified due to a lack of comparison studies in the literature.
We excluded the case reports, case series with fewer than 5 patients, review articles, abstracts, presentations without full-text availability, and the studies that were not published in English.
Two authors conducted the screening process, and any conflicts during this process were resolved with the senior author.
Risk of Bias
To evaluate the potential bias in the observational, nonrandomized studies included in our analysis, we used the Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I) tool 4. The tool assesses 7 distinct domains of bias, including confounding, selection of participants in the study, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of the reported result. Our assessment yielded an overall judgment of the risk of bias, which we categorized as low, moderate, serious, or critical.
Data Extraction
Data were extracted from each eligible study into an Excel 2021 spreadsheet (Microsoft). Study characteristics, baseline characteristics of the patients, and outcomes of interests were extracted from the studies after the screening process was completed. Outcomes of interest were: angiographic success, complications, vasospasm recurrence, and neurologic improvement. A qualitative assessment of each outcome was also performed.
Statistical Analysis
All statistical analyses were performed by using R software (R Foundation for Statistical Computing, Version 4.4.1) with the “meta” package. Additionally, the robvis package was used for visualizing the risk of bias assessment results.15
Data on outcomes of interest from each study were consolidated to compute the pooled prevalence (%) and their corresponding 95% CI, by using a random effects model due to the heterogeneity among the included studies in institutional protocols, reporting methods, and follow-up techniques among the studies. Moreover, we used the generalized linear mixed models method for better stability with small studies and rare events.16,17 For outcome(s) with rare events, we used double arscine transformation for rare events (eg, complication rate). Additionally, pre-established subgroup analyses for angiographic success were carried out for the type of device used (SR or ES). Heterogeneity was assessed by using Q-statistics and I2 test. The heterogeneity was considered significant when P < .05 or the I2 value was greater than 50%. In case of significant heterogeneity, a leave-one-out sensitivity analysis was performed to show impact of excluding single studies on both pooled effect size and heterogeneity. Although not optimal when the number of included studied is less than 10,18 we performed test of a publication bias by using funnel plots and Egger regression.
RESULTS
Search and Screening Results
The search strategy gave a total of 1209 studies, after removing duplicates the remaining 600 studies were further screened. There was a total of 27 studies selected for the full-text screening. Eventually, 8 studies were determined to satisfy our inclusion criteria with the appropriate report of outcomes of interest and data were then extracted from these studies (Supplemental Data).13,14,19⇓⇓⇓⇓-24
Study Characteristics and Risk of Bias
All 8 included studies used a single-arm intervention design; 6 were retrospective, 2 were prospective, 3 were multicenter trials, and 5 were single-center studies. The size of the included studies ranged from 6 to 40 patients, resulting in 14 to 129 targeted vessels. Through the 8 full-text articles, a total of 156 patients were collected, for 428 targeted vessels.
Low risk of bias was found in 3 studies, while moderate and serious risk of bias were found in 2 studies each; the last study had a critical risk of bias due to bias in the selection of participants. Domains of most concerns across studies were: bias due to confounding, bias due to the selection of participants, bias due to deviations from intended interventions, and bias in selection of the reported result (Fig 1).
Results of the methodologic quality assessment of the included studies based on ROBINS-I tool. A, “Traffic light” plots of the domain-level judgments for each individual result. B, Weighted bar plots of the distribution of risk of bias judgments within each bias domain.
Patients and Procedural Characteristics
Patient characteristics, including age, sex, Fisher and Hunt and Hess scores, and device used are detailed in the Supplemental Data. Vasospasms were occurring following subarachnoid hemorrhage due to aneurysm rupture in most patients (overall 147/156 [94.2%]). Angioplasties were mostly performed on the proximal intracranial arteries (ie, internal carotid artery, M1, V4, basilar artery, P1; overall 308/428 [72.0%]); while the study by Thiery et al23 only included distal segment arteries (ie, M2, M3, A2, A3, and P2).
An SR, deviated from its original purpose of mechanical thrombectomy, was used in 6 studies. An ES was used in 4 studies; both an SR and ES devices were used in 2 studies but were not compared. All 8 studies used intra-arterial vasodilator, before, during, or after the angioplasty. In the study by Kwon et al,14 patients were separated into 2 groups depending on whether the vasodilator was infused before or after the angioplasty.
For SR, a large panel of devices was used, including Trevo (Stryker), Solitaire (Covidien), NeVa (Vesalio), Catch/Catch-Mini (Balt), Aperio device (Acandis), pRESET devices (Phenox), and Tigertriever (Rapid Medical). For ES, only 2 devices tested met our inclusion criteria: the Comaneci (Rapid Medical) and the pRELAX (Phenox). The main difference between these 2 devices is that the Comaneci is a manually controlled expanding device while the pRELAX is similar to an SR but with a homogeneous radial force along the device.
Outcomes
Angiographic Success.
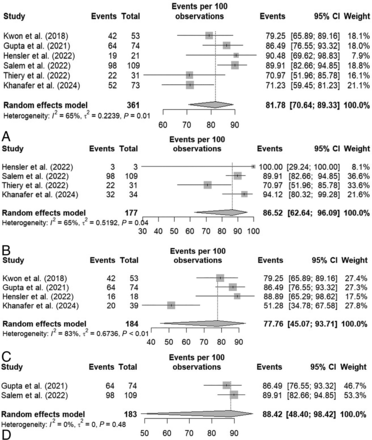
Six studies involving 361 targeted vessels presented the rate of angiographic success after mechanical angioplasty. The analysis indicated a rate of 81.8% (95% CI: 70.6–89.3). Notably, the heterogeneity was high among the studies (I2 = 65%, P = .01; Fig 2A). Leave-one-out sensitivity analysis did not detect any outlier study (Supplemental Data).
Forest plot of the random effects proportion meta-analysis of the reported rates of overall success rate (A), ES subgroup (B), and SR subgroup (C). Sensitivity analysis by using only prospective, multicenter studies (D).
Subgroup analysis with the type of device showed a similar rate with ES compared with SR: ES included 177 target vessels in 4 studies, yielding an angiographic success rate of 86.5% (95% CI: 62.6–96.1), while in the SR subgroup a total 184 target vessels were included in 4 studies, giving an angiographic success rate of 77.8% (95% CI: 45.1–93.7). Both subgroup analyses showed heterogeneity, (I2 = 65%, P = .04 and I2 = 83%, P < .01, respectively; Fig 2B, -C). Finally, we performed a sensitivity analysis by using only multicenter and prospective studies; the subgroup included 183 target vessels in 2 studies, yielding comparable result of angiographic success rate of 88.4% (95% CI: 48.4–98.4). No heterogeneity was noted (I2 = 0%, P = .48; Fig 2D).
It should be noted that different thresholds of persisting stenosis were used to define angiographic success, but <50% was the most applied definition. Studies also reported through different calculation the percentage of stenosis improvement after angioplasty, making it not comparable between studies.
Device Complications.
The meta-analysis of all 8 studies evaluated the rate of device-related complications in a total of 427 target vessels. The analysis revealed a rate of 1.1% (95% CI: 0.0–3.6). No significant heterogeneity was detected among these studies (I2 = 30%, P = .19; Fig 3A).
Forest plot of the random effects proportion meta-analysis of the reported rates of complication rate (A), recurrence rate (B) and neurologic improvement rate (C).
Complications reported were mainly per-procedural clot formations (6 cases) and 3 ruptures; of note, 2 ruptures of these 3 cases were secondary to the use of a balloon angioplasty after failure of the SR/ES device, and 1 was most likely due to microwire puncture during navigation.
Recurrence of Vasospasm.
In 7 studies involving 414 targeted vessels, the rate of vasospasm recurrence was 12.8% (95% CI: 5.2–28.1). Heterogeneity was observed among these studies (I2 = 78%, P < .001; Fig 3B). Leave-one-out sensitivity analysis did not detect any outlier study (Supplemental Data). It is important to note that vasospasm recurrence was a subjective outcome, because it was defined by the need for retreatment of the target vessel.
Neurologic Improvements and Final mRS.
Three studies including 59 patients reported the rate of postprocedural neurologic improvement (defined by a partial or complete resolution of the neurologic symptoms after the procedure). The analysis indicated a rate of 65.9% (95% CI: 51.1–78.1). No heterogeneity was noted among the studies (I2 = 0%, P = .77; Fig 3C).
Imaging techniques were used to further analyze delayed cerebral blood circulation and determine improvement/worsening/stability after the angioplasty. Lopez-Rueda et al19 used evaluated cerebral circulation time on digital subtraction angiography. They showed that 10 of 11 patients (90.9%) showed improvement in the cerebral circulation time. Gupta et al13 used the ASPECTS; mean ASPECTS score before treatment was 8.5 ± 1.5 (n=30) and at day 21 or discharge was 8.1 ± 2.0 (n=24), which they interpreted as a prevention of infarct progression posttreatment. Finally, Thiery et al23 were able to determine a rate of 16.7% (3/18) of delayed cerebral ischemia in the territory of the target vessel by using MRI or CT evaluation during follow-up.
In 4 studies, mRS at discharge was presented (mean ± standard deviation [n patient]): 2.3 ± 2.3 [12] (Kwon et al14), 3.1 ± 1.7 [30] (Gupta et al13), 3.7 ± 1.5 [12] (Hensler et al21), 3.9 ± 1.4 [25] (Khanafer et al24). Final mRS was available in 2 studies: 2.3 ± 2.3 [12] (Hensler et al21) and 2.8 ± 1.9 [25] (Khanafer et al24).
Publication Bias.
The Supplemental Data exhibit the funnel plots for each respective outcome as outlined in the Results section. These plots reveal no significant asymmetry for the outcomes of angiographic success, complication, and neurologic improvement. Significant asymmetry was noted for the recurrence outcome. This asymmetry suggests the potential presence of bias, which may be attributable to small study effects or publication bias.
DISCUSSION
This systematic review and meta-analysis provided evidence supporting stent angioplasty with SR and ES as a promising treatment option for cerebral vasospasm. Notably, SR and ES devices consistently achieved high angiographic success and low complication rates. Figure 4 presents a case illustration of stent angioplasty with ES.
Case illustration of stent angioplasty by using a manually controlled ES (Comaneci) to treat cerebral vasospasm. A, Antero-posterior angiogram of the posterior circulation of a patient recently treated for a ruptured basilar aneurysm with coils. Severe vasospasm of the basilar artery and both posterior cerebral arteries is depicted. B, Angiogram poststent angioplasty of the basilar artery (C), the right (D) and the left (E) posterior cerebral arteries shows complete or near-complete regression of the vasospasm in all 3 target vessels. White arrowhead in unsubtracted angiogram (C, D, and E) highlights the opening of the stent.
Currently, there are no clear guidelines for preventing delayed cerebral ischemia in patients with cerebral vasospasm due to aneurysmal subarachnoid hemorrhage.25 For instance, a recent meta-analysis by Ma et al26 showed this population had reduced in-hospital mortality with endovascular therapy. The positive results were mainly attributed to the subgroup by using intra-arterial vasodilators. However, no significant improvement was observed in long-term prognosis or functional recovery.26 Overall, the use of intra-arterial vasodilators is a safe treatment, but with variable efficacy on cerebral perfusion27 or functional recovery.26 Most importantly, the effect of chemical angioplasty is temporary, and in many cases, it fails to achieve adequate vessel dilation, with frequent recurrence of vasospasm, often necessitating multiple treatments.28 As a result, balloon angioplasty, combined with intra-arterial vasodilator infusion, is frequently proposed as a more long-lasting treatment, particularly in cases of severely narrowed proximal vessel segments.9 Recently, 2 separate studies reported that cerebral infarction related to angioplasty can occur in approximately 6% of treated patients.10,29 Although successful treatment with this technique can reduce the need for retreatment, the risk of complications remains a major concern.30
The use of SR and ES devices appears to be a safe alternative strategy for mechanical angioplasty associated with chemical angioplasty. Here, the number of complications reported was low (1.1% of all target vessels), with no heterogeneity detected. Moreover, complications due to thromboembolic events were successfully managed with intra-arterial injection of thrombolytic drugs; while hemorrhagic complications were reported as secondary to balloon angioplasty after failure of the expanding device23 or to microwire puncture during navigation.14 In the former situation, the number of repeated angioplasty maneuvers and over dilation may have caused the vessel rupture.
SR/ES devices maintain anterograde blood flow, avoiding the temporary cessation caused by balloons. This provides the advantage of keeping the device in the spasmodic vessels for more extended times than balloons; repeated inflations of a balloon and flow arrest in a cerebral artery have been shown to decrease brain tissue oxygenation.31 Another advantage is that vasodilators can be injected during the deployment of the stent, allowing the drug to reach the distal vasculature. It should be noted that all included studies used stent angioplasty in combination with intra-arterial vasodilator. In the study by Kwon et al,14 this strategy yielded better angiographic outcomes with fewer vasospasm recurrences compared with the use of vasodilators first (though not statistically significant). Previous in vivo studies with balloon angioplasty have shown that arterial paralysis can be achieved with less stretch force if the vessel is in a contracted state. For this reason, in our opinion, mechanical dilation should be performed before chemical angioplasty.32
Distal target vessels represented 28% of the total cohort in our analysis. Thanks to the advent of mechanical thrombectomy a decade ago, the experience of neurointerventionists with SR is high. Physician familiarity with and trackability of these devices makes distal stent angioplasty accessible and safe as well. More distal territories are less amenable to balloon dilation, as fatal arterial ruptures during balloon dilation have been reported.29
Our results suggest good angiographic efficacy of stent angioplasty. However, the results were heterogeneous. Although this can be explained by the heterogeneity of the outcome measured, it can be also secondary to the large variety of SR used. Many different types of retrievers are available on the market, and none of them was specifically designed for arterial angioplasty. When choosing an SR model, various factors must be considered. The radial force of the retriever depends not only on its size and design but also on the additional pressure applied by the operator during deployment and oversizing.33 Bhogal et al34 were also able to establish, by using mechanistic mathematical modeling, that dilation of vascular smooth muscle cells beyond a threshold of mechanical failure is sufficient to resolve cerebral vasospasm without damage to the underlying extracellular matrix, and that existing SR devices have sufficient radial force if adequately sized.
Finally, in this study we observed a similar angiographic outcome with ES (86.5%) compared with SR (80.5%). Two types of ES were included in this study: the Comaneci and the pRELAX. The main difference between these 2 devices is that the Comaneci is a manually controlled expanding device while the pRELAX has a similar design to an SR with a homogeneous radial force along the device. Bhogal et al12 also presented a case series of 4 patients safely and efficiently treated with a Cascade device (Perflow Medical), which is another manually controlled expanding device; they showed that the devices exhibited higher radial force than the Solitaire SR. Similar findings were shown by Salem et al22 with the Comaneci device. One interesting feature of the ES devices is that these devices “collapse” and the radial force decreases if one continues to expand them after reaching a maximum, which suggests an inherent unexpected safety feature.12
Our study has several limitations. Some reviewed studies had small sample sizes, affecting generalizability and statistical power. Disparities in the devices tested, intra-arterial vasodilators used, and outcomes reported may also limit the generalizability of the results. Moreover, different “techniques” were sometimes used: for instance, Khanafer et al24 used another technique referred to by the authors as Stent-ReLACSS, which includes a retrieval of the device. This technique introduces potentially further bias in the interpretation of the results. Single-arm designs without control groups may introduce confounding factors. Our analysis is also hindered by the vulnerability to bias in the included studies, with moderate to critical concerns in 5 of them. Concerns arise regarding the selection of participants and the presentation of results, which often lack robust measures to address these issues. Additionally, the absence of comprehensive demographic and baseline clinical data in studies makes it challenging to consider potential factors influencing outcomes at the individual level. Moreover, due to the small number of included studies, funnel plots and Egger regression tests of bias have a limited reliability.
CONCLUSIONS
This systematic review and meta-analysis on stent angioplasty in combination with intra-arterial vasodilator by using SR and ES devices suggests that it may be a promising treatment option for cerebral vasospasm. It demonstrates high rates of angiographic success and low incidences of adverse events. However, due to significant heterogeneity among the studies—in terms of protocols, devices, and drugs used—as well as serious biases in reporting results, these conclusions should be interpreted with caution. Future randomized controlled trials are needed to confirm the efficacy and safety of this approach compared with medical management and balloon angioplasty treatments.
Footnotes
J.C. received educational grants from The French Society of Neuroradiology (SFNR), the French Society of Radiology, The Philippe Foundation, and The Foundation Thérèse and René Planiol.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received September 12, 2024.
- Accepted after revision November 7, 2024.
- © 2025 by American Journal of Neuroradiology