Graphical Abstract
Abstract
BACKGROUND AND PURPOSE: Brachial neuritis is a monophasic condition affecting the brachial plexus and its branches, manifesting as acute shoulder and upper arm pain, followed by weakness and paresthesias. It can be triggered by antecedent events, including procedures such as surgery. Misdiagnosis and delay in diagnosis are common. Imaging is important to confirm the diagnosis of postprocedural brachial neuritis and exclude other etiologies.
MATERIALS AND METHODS: Clinical, electrodiagnostic, and neuroimaging features of patients with postprocedural brachial neuritis from a single quaternary care institution were identified and analyzed.
RESULTS: Six patients (2 women) were identified with a median age of 62 (range 49–70) years. Antecedent procedures included 4 cervical spine surgeries, 1 rotator cuff repair, and 1 central venous catheter placement. Time to symptom onset ranged from 1 day to 2 weeks. The initial symptom for 5 of the 6 patients was severe upper extremity pain followed by weakness. All patients had electrodiagnostic tests and MR neurography consistent with brachial neuritis. MR neurogram showed plexus and/or terminal branch abnormalities with associated muscular denervation edema. The C5 or C6 root and/or upper trunk were always involved. The most common branches affected were the suprascapular, long thoracic, and axillary nerves. Hourglass constrictions (HGCs) of these nerves were seen in 3 of 6 patients. The average time to diagnosis was 3.4 (range 1.5–5) months.
CONCLUSIONS: Postprocedural brachial neuritis is an under-recognized cause of acute upper extremity pain and weakness. MR neurography can exclude iatrogenic causes and document the presence of HGCs in affected nerves. Diagnostic neuroradiologists should be aware of this clinical entity and associated neuroimaging findings.
ABBREVIATIONS:
- ACDF
- anterior cervical discectomy and fusion
- EDX
- electrodiagnostic
- HGC
- hourglass constriction
- MUAP
- motor unit action potential
SUMMARY
PREVIOUS LITERATURE:
Brachial neuritis, characterized by profound pain followed by weakness in the distribution of the brachial plexus, is reported to be preceded by surgery in 10%–14% of cases. Previous studies have described various imaging and electrodiagnostic characteristics associated with this condition, demonstrating that lesions may involve either the plexus itself or its terminal branches. Given the rarity of this condition, misdiagnosis is common. This may be particularly true for postprocedural patients, where the etiology of their symptoms could also be due to procedural or idiopathic complications, such as C5 palsy.
KEY FINDINGS:
We describe the clinical course, electrodiagnostic, and imaging findings in 6 patients with postprocedural brachial neuritis. MRI revealed supraclavicular plexus involvement in all patients. HGCs of terminal nerve branches were present in 3 of 6 patients. MR neurogram enabled accurate diagnosis and exclusion of iatrogenic causes in all patients.
KNOWLEDGE ADVANCEMENT:
Recognition of postprocedural brachial neuritis is important for surgeons, neurologists, and radiologists as it may mimic iatrogenic neurologic deficits, further delaying diagnosis. Our study advances our understanding of postprocedural brachial neuritis by describing its clinical, electrodiagnostic, and imaging characteristics, potentially aiding in more rapid and accurate diagnosis.
Brachial neuritis (neuralgic amyotrophy, Parsonage-Turner Syndrome) is a subacute, monophasic neurologic condition affecting the brachial plexus and its branches, classically manifesting as acute pain in the shoulder girdle and upper arm followed by weakness and paresthesias.1 Brachial neuritis is often triggered by an antecedent event, including surgery.2,3 Procedures associated with brachial neuritis include knee surgery,4 cervical surgery (most common),5⇓–7 mastectomy,8 appendectomy,9 tonsillectomy,9 cardiac or rib surgery,9 hysterectomy,9 and laparoscopic treatment of endometriosis.10 A common postcervical surgery phenomenon is C5 palsy, which is postulated to be due to brachial neuritis.5 Postprocedural brachial neuritis is reported to manifest within a week to months following surgery but can occur within 24 hours of surgery.
Misdiagnosis and delay in diagnosis of brachial neuritis are common.4 Imaging guided by clinical findings can help distinguish this diagnosis from other etiologies, which may be particularly helpful in postprocedural patients where the etiology of their symptoms could be due to other causes, such as a procedural complication.11 Characteristic imaging findings of brachial neuritis may include intrinsic constrictions of involved nerves (Fig 1) and/or denervated muscles around the shoulder.5⇓–7
A 48-year-old man with acute right shoulder pain and weakness and clinically suspected idiopathic brachial neuritis. Axial T2 IDEAL image (GE Healthcare) from a brachial plexus MR neurogram shows increased signal and caliber of the right suprascapular nerve with multiple constrictions (arrows) along its course, confirmatory imaging findings of brachial neuritis. Denervation edema involving the supraspinatus and infraspinatus muscles (asterisks) is seen.
In this study, we present a case series of 6 postprocedural brachial neuritis patients and their electrodiagnostic (EDX) and imaging findings. Awareness of brachial neuritis as a postprocedural sequela is important because early detection can allow for appropriate management and treatment of patient symptoms and improve patient outcomes.
MATERIALS AND METHODS
We retrospectively reviewed clinical, electrodiagnostic, and imaging features of patients diagnosed with brachial neuritis seen at a single tertiary care center from July 2021 to March 2023 to identify patients with a procedure as an antecedent event. Patients were included if they met inclusion/exclusion criteria for brachial neuritis (Table 1), developed symptoms of brachial neuritis within 2 months of the procedure, and underwent MR neurography (protocol described in Table 2) following the development of brachial neuritis symptoms. MR neurograms were performed on a 3T MRI scanner (Signa Premier or Discovery 750; GE Healthcare) and were reviewed by 2 neuroradiologists with a combined experience of greater than 25 years. Patients were scanned in a conventional manner with arms in a neutral position. Specific imaging features that were assessed included the presence or absence of intraneural edema, nerve constrictions, denervation edema, and evidence of postoperative collections causing mass effect. All patients consented to participation in our institution’s Brachial Neuritis Registry (Institutional Review Board 21–34564, expedited review) and have agreed to the use of their deidentified information for this case series. The methodology described by the STROBE checklist for observational studies was followed.
Brachial neuritis inclusion and exclusion criteria
MR neurography protocol for the brachial plexusa
RESULTS
Six (2 women, with a median age of 62 [range 49–70] years) out of 68 patients (8.8%) diagnosed with brachial neuritis at our center had a procedural antecedent event and had MR imaging as part of their evaluation. The rate of postprocedural brachial neuritis in our center’s registry during the same time was 13.2%. Five patients were included based on clinical criteria described in Table 1. In 1 patient (BN3), the clinical criteria were equivocal (moderate instead of severe pain), but the imaging criteria were definitive (Table 1). Antecedent procedures included 4 cervical spine surgeries (2 multilevel anterior cervical discectomy and fusions [ACDFs], 1 multilevel posterior fusion with laminectomies, 1 multilevel disc arthroplasty), 1 rotator cuff tear repair, and 1 intrajugular central venous catheter placement. All patients presented with brachial neuritis for the first time. The median time to accurate diagnosis was 3.75 (range 1.5 to 5) months, and the median number of clinicians seen before accurate diagnosis was 4.5 (range 2 to 7).
Each patient’s clinical presentation, electrodiagnostic features, and neuroimaging findings are described in detail in the Supplemental Data. The median time between the procedure and symptom onset was 4.5 days (range 1 day to 2 weeks). The initial symptom for 5 out of 6 patients was severe upper extremity pain followed by weakness. One patient (BN3) presented atypically with weakness followed by moderate pain. Scapular winging was present in all but 1 patient (BN 1). The next most common affected movement was external rotation of the shoulder in 4 of 6 patients. Finger extension was weak in 4 of 6 patients, and shoulder abduction in 2 of 6 patients.
Electrodiagnostic studies were performed on all patients. The specific protocol for the nerve conduction studies and electromyography was selected by the electromyographer based on the clinical information. In 5 of the patients tested, the results were consistent with the clinical diagnosis of brachial neuritis (Supplemental Data). A limited EDX study was done for 1 patient (BN3) at 1 year to assess nerve continuity to the most affected muscles, which is an important prognostic sign. One patient (BN10) had superimposed bilateral mild median neuropathies at the wrist (carpal tunnel syndrome) and 1 (BN7) had a moderate superimposed right median neuropathy at the wrist.
All patients had weakness at the time of imaging. The pain had resolved at the time of imaging in 2 patients (BN2, BN10). Imaging showed plexus and/or terminal branch abnormality with associated muscular denervation edema in all 6 patients (Supplemental Data) that closely correlated with EDX features. The supraclavicular plexus, particularly the C5 root, C6 root, and/or upper trunk, was always involved (Figs 2–4). Branch involvement was variable, with suprascapular, long thoracic, and axillary nerves being most affected by imaging. Hourglass constrictions (HGCs) along the course of the involved branches were seen in 3 of 6 patients (Figs 2, 3). Mild postcontrast enhancement was noted along the affected nerves in most cases. Enhancement of the denervated muscles was always present. Imaging was important for the diagnosis and management of all patients. BN1 had a myelodysplastic syndrome with the need to exclude malignant infiltration. For all other patients, excluding an iatrogenic cause related to the antecedent surgery, such as hematoma or infection, was important for diagnosis and appropriate management. In 2 patients (BN8 and BN9), where follow-up imaging was obtained, repeat imaging several weeks out from the initial study showed improvement in muscle denervation and intraneural edema compared with the first imaging study. This was consistent with gradual clinical improvement in both patients.
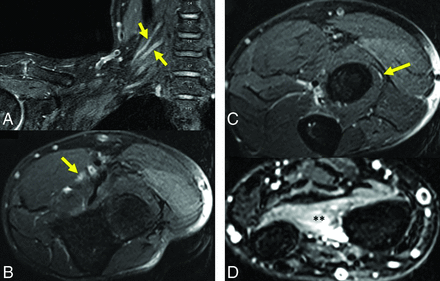
A 58-year-old man (BN2) with onset of right brachial neuritis 1-week postop from right rotator cuff repair. MR neurogram was performed 4 months after initial symptom onset for persistent right upper extremity weakness. A, Coronal T2 IDEAL sequence from right brachial plexus neurogram shows intraneural edema of the right C5 and C6 roots (arrows). B, Axial T2 IDEAL sequence from a right upper extremity neurogram shows increased fascicular signal of the median nerve (arrow) between the 2 heads of the pronator teres, as well as increased signal of the posterior interosseous nerve (arrow) as it courses through the supinator muscle (C). Denervation changes within the flexor and extensor compartments of the forearm, including diffuse edema within the pronator quadratus muscle (asterisks in D), which is supplied by the anterior interosseous nerve.
A 66-year-old woman (BN3) with onset of right brachial neuritis 2 weeks after a C4-C7 ACDF. MR neurogram of the right brachial plexus was performed 5 weeks after initial symptom onset. A, Coronal T2 IDEAL sequence shows diffuse intraneural edema of the right brachial plexus, particularly C5-C8 roots (dashed oval), upper trunk (solid arrow), and posterior cord (dashed arrow). ACDF hardware (asterisks). B, Sagittal T2 IDEAL sequence shows increased signal of the suprascapular nerve (arrow) and denervation edema within the supra- and infraspinatus and trapezius muscles (asterisks). C, Sagittal T2 IDEAL image more proximal than (B) shows increased signal of the long thoracic nerve. Constrictions were noted along the course of the long thoracic and suprascapular nerves (now shown).
A 68-year-old man (BN8) with onset of left brachial neuritis 2 days after C2-C6 laminectomies and C2-C7 posterior spinal fusion. MR neurogram of the left brachial plexus was performed at 3 days, 5 weeks, and 7 months after initial symptom onset. A, Coronal T2 IDEAL sequence from neurogram performed at 5 weeks shows intraneural edema involving the left C5-C8 nerve roots with extension into the upper and middle trunks and lateral and posterior cords. Constrictions along the course of the suprascapular (arrow in A) and long thoracic (arrow in B) nerves. Intraneural edema involving the affected nerves continued to improve over the serial imaging studies.
Pain responded substantially to corticosteroids in 2 patients. Four received opioids with partial benefit in 1. Two received NSAIDs with partial benefit. Three patients received a neuropathic pain medication (gabapentin and/or a tricyclic antidepressant) or muscle relaxant (baclofen, tizanidine) with unclear benefit. Four patients received physical and occupational therapy through our Brachial Neuritis Multidisciplinary Clinic with benefit. The other 2 patients received physical therapy before coming to our center with improvement, particularly in range of motion.
All experienced at least partial pain relief by a median of 2 months following symptom presentation (range 1 week to 9 months). Only 1 patient (BN9) had persistent pain exceeding 1 year following symptom onset. All patients had improvement in their weakness over the course of 10 to 24 months (Supplemental Data), but the recovery was incomplete, with mild to moderate persistent weakness in all patients.
DISCUSSION
Postprocedural brachial neuritis is an under-recognized clinical entity with undue delay in diagnosis, with most cases ascribed to brachial plexus stretch injuries occurring during anesthesia or direct injury as a result of the procedure. In Parsonage and Turner’s original description of brachial neuritis, 10% of patients had antecedent surgery 3–14 days before symptom onset.1 In another study by Malamut et al,9 6 patients, 1–13 days postprocedurally, developed signs and symptoms that met the clinical and electrophysiologic criteria for brachial neuritis. The largest published series of 246 patients with brachial neuritis showed surgery as an antecedent event in 14% of patients.3 The incidence of postprocedural brachial neuritis in our center’s registry is similar (13.2%). Our study confirms the challenge with recognition and accurate diagnosis of brachial neuritis in the postprocedural period. While the diagnosis of brachial neuritis is usually based on clinical criteria, electrodiagnostic and imaging findings are helpful for supporting the diagnosis (Table 1) and are particularly useful when definitive clinical criteria are not met or when there are confounding factors such as recent cervical spine surgery. Nonspecific antiganglioside antibodies are present in 36% of patients.12 However, they are not clinically useful in confirming the diagnosis or excluding other causes, and they were not performed in these patients.
EDX and MR neurography imaging findings are complementary and can confirm and localize the distribution of nerve involvement, exclude other neuromuscular causes, assess improvement over time, and select patients for surgical treatment. Imaging studies may show nerve abnormalities and muscle changes within hours to days of symptom onset, whereas a minimum 3–4 weeks interval from symptom onset is typically chosen to increase EDX sensitivity to ensure sufficient time for denervation changes to be present.13
There is conflicting data in the literature regarding the MR neurography findings in brachial neuritis. In 1 study of 15 patients with idiopathic brachial neuritis, based on clinical and electrophysiological findings, MR neurography demonstrated root (53.3% of cases), trunk (46.7%), cord (40%), and/or terminal branch involvement (13.3%). The C5 root was the most common nerve root involved, and the lateral cord was the most common cord involved. Muscle denervation changes in the form of edema, fatty infiltration, and/or atrophy were noted in 8 (53.3%) patients. Most of the patients in this study had unilateral involvement in MR neurography.14 However, in another retrospective study, Sneag et al15 characterized lesion distribution in 27 patients with brachial neuritis by using high-resolution MRI and did not note plexus involvement but focal constrictions of nerves off of the plexus as a major change on imaging with patients with brachial neuritis. In this study, all patients had at least 1 clinically involved nerve. MRI revealed that the plexus appeared normal in 24 of 27 patients; in 3 other patients, signal hyperintensity was seen immediately proximal to the takeoff of abnormal side or terminal branch nerves. Focal intrinsic constrictions were detected in 32 of 38 nerves. The authors concluded brachial neuritis (Parsonage-Turner Syndrome) is >characterized by 1 or more mononeuropathies rather than changes involving a portion of or the complete plexus proper. Additionally, Sneag et al16 observed HGCs along the involved terminal branches in 90.2% of patients of a total of 123 patients with brachial neuritis with 3T MR neurography performed within 90 days of EDX studies.
Our study of postprocedural brachial neuritis supports the involvement of the plexus and roots as the supraclavicular plexus, particularly the C5 root, C6 root, and/or upper trunk, was always involved (Figs 2 – 4). There was variable branch involvement with suprascapular, long thoracic, and axillary nerves most affected on imaging in one-half of the patients. Differences in imaging features of brachial neuritis between our study and other published studies, particularly with regards to plexus versus terminal branch involvement and incidence of HGC along involved nerves, may be due to several reasons. These include differences in patient population (all our patients had postprocedural brachial neuritis), sample size, differences in scan protocols, and/or imaging at different time points from symptom onset.
The cause of HGCs along nerves in patients with brachial neuritis is not well understood.17 During explorative surgeries, HGCs are characterized by a very focal constriction of a nerve or part of it (1 or more fascicles), usually associated with nerve thickening proximally and distally to the constriction.17 Histology reveals complete loss of myelinated fibers at the HGC site. It is not known whether the number and severity of constrictions correlate with impaired function or recovery. There is some evidence that neurolysis of HGC may result in improved outcomes.17⇓–19 Based on available evidence, it appears likely, however, that the identification of HGCs with MR neurography is specific for and supports a clinically suspected diagnosis of brachial neuritis.15
From the surgeon and neuroradiologist perspective, recognition of the clinical and imaging presentation of brachial neuritis is particularly important, as brachial neuritis may mimic iatrogenic neurologic deficits. Consideration of brachial neuritis in the differential diagnosis is important for surgeons who operate on the cervical spine or shoulder as this diagnostic consideration helps the surgeon to request relevant diagnostic and imaging tests and involve specialists from neurology and radiology. Sudden pain and weakness due to brachial neuritis following a procedure could be erroneously diagnosed as a surgical complication leading to loss of patient trust and misguided medical malpractice claims.
Appropriate diagnosis is also important to ensure the optimal treatment. If the diagnosis is made within 4 weeks, a course of oral corticosteroids is recommended, which can help improve patient pain.2 Intravenous immunoglobulin may be an alternative if corticosteroids are contraindicated.2 Targeted rehabilitation that focuses on proper movement of the shoulder and avoiding overuse can prevent or improve scapular dyskinesia and chronic pain.2 This is important as typical physical therapy for shoulder weakness focuses on increasing strength, which can worsen function in these patients. Unfortunately, the recovery process is lengthy, and many patients are left with residual pain or weakness. In our case series, while all patients experienced relative improvement, all continued to experience residual mild-moderate weakness at the last follow-up. In select cases, surgical intervention may be considered.19 Recurrence risk is important to discuss with patients, especially if they are considering another procedure and have concerns about another episode. The risk of a single recurrence is about 20% in all patients with brachial neuritis, regardless of whether an antecedent event was present.3
This study has several limitations, including its retrospective nature. Further, while this study represents one of the larger series focusing on postprocedural brachial neuritis and MR imaging, the series is limited by its small number of 6 patients. In addition, while we employed strict inclusion criteria, permitting brachial neuritis cases to be classified as postprocedural only if symptoms presented within 2 months, it is not possible to definitively attribute each case of brachial neuritis to a procedural antecedent event. Further, as recognition and diagnosis of brachial neuritis is difficult, the 6 patients in our series underwent various EDX protocols (selected by the electromyographer based on clinical presentation) and had MR neurogram imaging at various intervals in their disease course—these differences may have affected the findings that we describe. Finally, while we followed some patients in our series for multiple years, longer-term follow-up is required to better characterize the chronic disease course and recovery process.
CONCLUSIONS
Postprocedural brachial neuritis is an uncommon and under-recognized cause of acute upper extremity pain and weakness. MR neurography, with clinical guidance, can exclude iatrogenic and other causes of postprocedural upper extremity pain and weakness and document the presence of HGCs in affected nerves. These changes can occur early in the course before denervation changes are evidenced by EDX.
Early recognition may allow for appropriate treatment and interventions. Neurologists and surgeons, especially those who operate at and around the cervical spine and shoulder, should include postprocedural brachial neuritis in their differential for cases of upper arm/axial pain and weakness. Misdiagnosis of this idiopathic condition may lead to concerns of surgical complications and lead to inappropriate medical malpractice claims. Diagnostic neuroradiologists should be aware of this clinical entity and associated neuroimaging findings.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received July 26, 2024.
- Accepted after revision November 1, 2024.
- © 2025 by American Journal of Neuroradiology