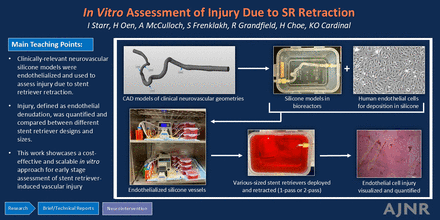
Graphical Abstract
SUMMARY:
Mechanical thrombectomy devices have potential to injure the vessel during treatment of acute ischemic stroke. The goal of the current work was to tailor in vitro endothelialized silicone models for stent retriever assessment and to evaluate endothelial injury following treatment by various stent retriever designs and sizes. Clinically relevant neurovascular geometries were first modeled out of silicone, then sterilized, coated with fibronectin, placed in bioreactors, seeded with human endothelial cells, and cultivated under flow. Several sizes of 2 different commercially available stent retrievers were then deployed in, and retracted through, vessels. Vessels were immediately harvested and stained. Endothelial injury, identified as denudation, was quantified by using ImageJ. Results illustrated that endothelial injury ranged from 16%–18% in wire/microcatheter-only treated vessels, 37%–61% in 1-pass treatments, and 52%–70% in 2-pass treatments. Overall, this work showcases an in vitro approach for early stage assessment of the extent and location of vascular injury following stent retriever retraction.
Stent-retriever thrombectomy is a well-established standard of care for treating acute ischemic stroke.1 The stent retriever is an expandable device that is deployed between the vessel wall and thrombus, then retrieved for thrombus extraction.2 Stent retrievers are chosen to treat acute ischemic stroke due to their effective retrieval of a wide spectrum of clot types, rapid restoration of blood flow, and favorable clinical outcomes.3
Although stent-retriever thrombectomy is effective, it is thought that the technique may induce device-related vascular injury.4,5 The potential risk of vascular injury is attributed to the outward force applied by the stent retriever to the vessel wall.6 The clinical significance of device-induced vascular injury in the form of endothelial damage remains unknown, and with constantly improving stent retriever devices, there is an ongoing desire to assess the potential for vascular injury.6⇓⇓⇓-10
Preclinical assessment of stent retriever induced endothelial injury is typically performed by using in vivo models.8,9,11 For example, Arai et al8 used a rabbit model to demonstrate that stent retrievers caused arterial wall injury extending into the medial layer and Gory et al11 evaluated risks ranging from denudation and medial layer edema to internal elastic lamina fracture in a porcine model. Similarly, stent retriever induced endothelial injury has been shown following canine neurothrombectomy procedures.9 In vitro models have not been commonly used to assess vascular injury; however, they have the potential to facilitate device assessment in a low cost and scalable model. An example from Teng et al12 demonstrated endothelial injury following stent retriever thrombectomy in an in vitro live-cell model. Results illustrated the pattern and degree of endothelial cell injury following mechanical thrombectomy in straight vessels.12
Recent advances with endothelialized silicone models have illustrated the ability to create more complex in vitro vessels to assess catheter induced endothelial injury.13 Specifically, prior work utilized a tortuous neurovascular vessel pathway to evaluate injury following guidewire, biaxial, and triaxial catheter use. However, stent retrievers were not evaluated. Therefore, the goal of the current work was to evaluate endothelial injury due to stent retriever retraction in complex endothelialized silicone models. The first step was to perform a pilot study, by using the same neurovascular pathway previously published, but this time evaluating stent retriever retraction. Following successful pilot use, 2 larger studies were performed in customized vascular geometries to further evaluate endothelial injury following retraction of different sizes and types of stent retrievers. For the 2 larger studies, stent retriever deployment and retraction were performed by a practicing neurointerventionalist, and injury results were compared overall by device type and between specific regions of interest.
MATERIALS AND METHODS
Three separate studies were performed, including the pilot study followed by the 2 larger main studies. Main study A focused on various-sized stent retriever deployment, while Main study B focused on 3 mm stent retriever deployment in vessels down to 1.5 mm. For all studies, human endothelial cells were cultured as described below, followed by vessel creation and cultivation. Vessels were then acutely treated with designated stent retriever types and sizes, followed by fixation and assessment of endothelial injury. Silicone vessel geometry and specific device treatments varied between the 3 studies, as described below.
Silicone Models and Bioreactor Systems
Neurovascular silicone models were designed and obtained from Stryker Neurovascular to recreate specific dimensions and tortuosity relevant to clinical stent retriever use. To create the silicone models, the desired geometry was first 3D printed out of acrylonitrile butadiene styrene. This 3D printed geometry was smoothed, then dipped in silicone. Finally, the acrylonitrile butadiene styrene was dissolved, leaving a patent lumen within the desired geometry.
The pilot study utilized a previously published tortuous neurovascular pathway,13 with an inner diameter tapering from 5.5 mm to 4.0 mm, as illustrated at the top of Fig 1A. The fabricated silicone model mounted into a bioreactor is shown in Fig 1B.
Custom neurovascular silicone geometries, vessel models, and device assessment. Geometries were first created in CAD (A), with the pilot study (top) utilizing the same tortuous neurovascular pathway previously published,13 while main study A (middle) and main study B (bottom) each incorporated new geometries relevant to stent retriever use in the cerebral vasculature. Dimensions, ranging from 5.5 mm proximal ends to 1.5 mm distal ends, are noted on the computer models. Silicone models were fabricated based on these custom geometries, then sterilized and coated with fibronectin. Coated silicone models were placed in bioreactor systems (B) before human umbilical vein endothelial cell deposition. Vessels were then cultivated on peristaltic pumps in a large incubator for 3–4 days before device treatment (C). On the day of treatment, stent retrievers were inserted into endothelialized silicone vessels, deployed and retracted, then harvested for evaluation (D). After retraction through the vessels (top), they were analyzed by using H&E imaging, which revealed the expected cobblestone morphology of endothelial cells in untreated controls (middle) and endothelial denudation from stent retriever induced injury (bottom).
Main study A utilized a new geometry with a proximal inner diameter of 5.5 mm to distal inner diameter of 2.5 mm. The computer aided design model of this geometry is illustrated in the middle of Fig 1A along with a photo of the resultant silicone within a bioreactor in Fig 1B.
Main study B incorporated a modified new geometry that featured an even smaller distal inner diameter. This geometry included a proximal inner diameter of 5.5 mm and a distal inner diameter of 1.5 mm. The computer model and the silicone mounted in a bioreactor are shown in the bottom of Fig 1A and -B.
Vessel Cultivation and Stent Retriever Use
Human umbilical vein endothelial cells (Lonza, C2519A) were expanded in culture at 37°C and 5% CO2. Silicone models were endothelialized by using previously described methods.13,14 Briefly, silicone models were sterilized in 70% ethanol, rinsed with 1× tris-buffered saline, coated with 20 μg/mL of fibronectin (MilliporeSigma F1141), incubated at 37°C and 5% CO2 for 1 hour, and aseptically mounted into individual sterile bioreactor systems. Bioreactor chambers were connected to media reservoirs and placed on Masterflex L/S Cole-Parmer 8-roller peristaltic pumps. EGM-2 media (Lonza, CC-3162) was circulated through the luminal path before cell deposition. Human umbilical vein endothelial cells were injected with a concentration of 4.0–6.3 million cells per milliliter, and bioreactor systems were then rotated. Low flow was initiated after 1 hour, then gradually increased to 90 revolutions per minute (4.7 mL/min) over 16 hours. A photo of vessels being housed in the incubator on peristaltic pumps is provided in Fig 1C.
Three to 4 days after cell deposition, designated bioreactor systems were removed from the incubator for stent retriever deployment. To maintain physiologic temperature, bioreactors were removed and treated 1 at a time, then returned to the incubator. Access to the deployment region was achieved by inserting a long sheath into the proximal end of the silicone model and tracking a Synchro2 Standard guidewire (Stryker) and Trak 21 microcatheter (Stryker) to the distal end of the vessel. After removal of the guidewire, the stent retriever was advanced through the microcatheter, then the microcatheter was unsheathed to deploy the stent retriever. After waiting 1 minute, the stent was retracted through the model and into the long sheath, constituting 1 “pass.” In a subset of vessels, this was repeated an additional time for the 2-pass treatments. Devices were deployed and retracted visually without the use of fluoroscopy or other imaging systems.
For the pilot study, 8 vessels were created and the following devices were deployed by a nonphysician user:
Trevo ProVue NXT 6 × 37 mm (Stryker) (n = 3)
Solitaire X 6 × 40 mm (Medtronic) (n = 3)
Untreated controls (n = 2)
For main study A, 21 vessels were created, then a practicing neurointerventionalist performed device deployments as follows:
Trevo ProVue NXT 3 × 32 mm, 1 pass (n = 3)
Trevo ProVue NXT 4 × 41 mm, 1 pass (n = 4)
Trevo ProVue NXT 6 × 37 mm, 1 pass (n = 3)
Solitaire X 6 × 40 mm, 1 pass (n = 3)
Trevo ProVue NXT 4 × 41 mm, 2 pass (n = 3)
Solitaire X 6 × 40 mm, 2 pass (n = 3)
Untreated controls (n = 2)
For main study B, 18 vessels were created, then the same neurointerventionalist returned for deployments, as follows:
Trevo ProVue NXT 3 × 32 mm, 1 pass (n = 3)
Trevo ProVue NXT 3 × 32 mm, 2 pass (n = 3)
Solitaire X 3 × 40 mm, 1 pass (n = 3)
Solitaire X 3 × 40 mm, 2 pass (n = 3)
Synchro2 Standard/Trak 21 only, 1 pass (n = 2)
Synchro2 Standard/Trak 21 only, 2 pass (n = 2)
Untreated controls (n = 2)
Following stent retriever deployment and retraction, vessels were harvested and fixed in 100% methanol then stained with H&E to visualize endothelial injury.
Staining and Imaging
Vessel segments were stained with H&E by submerging samples in hematoxylin, deionized water, and eosin, then rinsed in deionized water, as previously published.13,14
Quantification of Endothelial Cell Denudation following Stent Retriever Retraction
Endothelial injury, defined in this model as denudation of the endothelium, was characterized and quantified with H&E-stained segments by using ImageJ quantitative analysis, as previously described.13 Briefly, 3 to 6 images were obtained at 4× from each region’s H&E-stained longitudinal halves. The surface area of denudation was manually traced by using ImageJ and divided by the entire surface area to determine a percent vessel injury.
Data Analysis
The percent surface area injured was calculated for each longitudinal half, then averaged for each region of interest to calculate the average percent endothelial cell injury. Then, the average endothelial injury per region was averaged to calculate each vessel’s average endothelial cell injury. Data are represented as mean ± standard error.
RESULTS
Pilot Study
Silicone models were successfully endothelialized with consistent endothelial cell linings before device deployment. Stent retriever deployment and retraction was successfully performed in the silicone vessels (Fig 1D). The transparency of silicone vessels allowed for device deployment to be performed without the need for fluoroscopy, and vessel injury was visualized as denudation of the endothelial cell monolayer by using H&E (Fig 1D).
In the pilot study, ImageJ quantification of H&E images revealed an average endothelial injury of 30.85% ± 1.33% in Trevo ProVue NXT 6 × 37 mm 1-pass treated vessels (n = 3), 31.09% ± 1.82% in Solitaire X 6 × 40 mm 1-pass treated vessels (n = 3), and 3.73% ± 1.18% in untreated controls (n = 2).
Main Study A
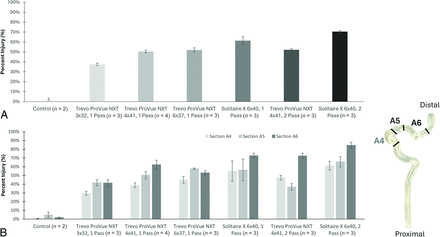
In main study A, injury ranged from <3% in control vessels to approximately 50%–70% in the 2-pass treatment groups. (Table; Fig 2A). Results were further analyzed by primary regions of interest (Fig 2B) with the highest injury typically observed in the most distal region.
Main study A evaluation of Trevo ProVue NXT (3 × 32 mm, 4 × 41 mm, 6 × 37 mm) and Solitaire X (6 × 40 mm) stent retriever-induced endothelial injury after 1- or 2-pass treatment. Endothelial injury ranged from <3% surface area injured in control vessels to approximately 50%–70% surface area injured in the 2-pass treatment groups (A). In the main study A geometry, results from 3 regions of interest, identified as A4, A5, and A6 in the labeled diagram, illustrated that higher injury was most often observed in the distal narrow region of the vessels (B).
ImageJ quantification of H&E images from main study A and main study B revealed the average % surface area injured for each treatment type as listed
Main Study B
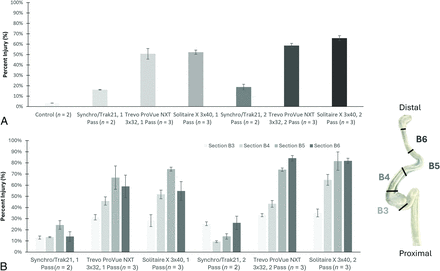
In main study B, injury ranged from <4% in control vessels, to 16%–18% in vessels treated with wire/microcatheter alone, to 58%–66% in the 2-pass treatment groups (Table; Fig 3A). Results were further analyzed by region (Fig 3B) and the highest injury was most often observed in the narrow distal regions.
Main study B evaluation of endothelial injury induced by Trevo ProVue NXT 3 × 32 mm and Solitaire X 3 × 40 mm stent retrievers after 1- or 2-pass treatment. Wire/microcatheter only treatments were also included as a baseline measurement for comparison. Vascular injury ranged from <4% surface area injured in control vessels, to 16%–18% surface area injured in wire/microcatheter treatments, to 58%–66% surface area injured in the 2-pass treatment groups (A). In the main study B geometry, results from 4 regions of interest, identified as B3, B4, B5, and B6 in the labeled diagram, illustrated that higher injury was typically observed in the narrow distal regions of the vessels (B).
DISCUSSION
This work illustrates the use of endothelialized silicone models for assessment of endothelial injury following stent retriever deployment and retraction. The pilot study established initial feasibility while main study A and main study B implemented new clinically relevant geometries with smaller distal regions and tested a wider range of device types and sizes. Stent retriever deployment and retraction in main studies A and B was performed by a neurointerventionalist, ensuring consistent and clinically relevant device use, and results were delineated between specific regions of interest.
Overall, results illustrated expected trends. Stent retriever retraction induced more injury than wire/microcatheter-only treatments, and 1-pass treatments tended to cause less injury than corresponding 2-pass treatments. Main study A included a range of device sizes, and vessels treated with the smallest device (Trevo 3 × 32 mm) exhibited significantly lower injury overall than the larger devices. Additionally, based on regional analysis, the narrower distal regions tended to experience higher injury. These results provide data about the extent and location of injury caused by the present devices, and support the future use of these models for comparing vascular injury and assessing interaction between device size and vessel size for additional device configurations and novel prototypes.
Although a prior publication demonstrated the ability to assess stent retriever injury in vitro,12 the vessels were short, straight segments. The present studies built on prior work by incorporating several key advances. First, the silicone geometries presented here are more clinically relevant than straight constructs. The assessment of endothelial injury by region also allowed for comparison of injury in regions with increased tortuosity or smaller diameters. These advancements provide a new option for early-stage preclinical assessments.
Clinical data has been published indicating that vascular injury is a concern following stent retriever thrombectomy.4,5 Human cadaver studies can provide a clinically relevant environment but do not typically allow for histologic assessment of treated vessels. A “live cadaver” model may allow for vascular injury assessment, but stent retriever induced vascular injury has not been shown.15 More commonly, animal models are used to provide data on biologic metrics such as vascular injury, and studies have demonstrated that thrombectomy can induce endothelial injury.8,9 However, animal models are generally limited in their ability to represent the vascular geometric complexity observed in humans and require extensive infrastructure and imaging that can be costly to perform.
In vitro models have the opportunity to combine clinically relevant geometries with key aspects of the cellular environment by using cost-effective, consistent, and scalable techniques. However, there are several limitations. First, the in vitro models presented are composed only of a monolayer of endothelial cells, making it impossible to induce or assess injury that extends deeper into the internal elastic lamina, medial, and adventitial layers, or to evaluate subsequent events such as acute vessel thrombosis and delayed stenosis. This means that the model presented will be complementary to (as opposed to a complete replacement of) other preclinical models. Additionally, the interaction of catheters and devices with endothelialized silicone vessels is not the same as endothelium on matrix in a native vessel, and these differences have not been fully characterized. However, trends and results illustrated here suggest that the in vitro model is useful for assessing differences in device-induced endothelial injury and making comparisons between device designs, sizes, and configurations. Future work will focus on direct comparisons to standard in vivo models, and future model customization may involve creating more complex models with medial smooth muscle layers or more physiologic flow parameters.
Overall, the current work illustrates that clinically relevant endothelialized silicone models can be used to evaluate endothelial denudation following retraction of different stent retriever designs and sizes, showcasing a novel in vitro approach for early-stage preclinical assessment of stent retriever–induced vascular injury.
Acknowledgments
We would like to acknowledge Jonah Adams for his assistance with vessel staining, Brianna Yang and Meili Laiho for their assistance with image tracing, and Jack Dooley for his assistance with the endothelial cell culture in these studies.
Footnotes
Isabelle Starr, Harrison Oen, and Alyssa McCulloch contributed equally to this article.
This work was funded by Stryker Neurovascular.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received June 11, 2024.
- Accepted after revision September 8, 2024.
- © 2025 by American Journal of Neuroradiology