Abstract
BACKGROUND AND PURPOSE: Cerebral vasospasm remains a strong predictor of poor outcomes after aneurysmal subarachnoid hemorrhage (aSAH). Endovascular treatment of vasospasm can be considered when conservative treatment options are exhausted, but its superiority over standard treatment remains a subject of critical debate. This study focuses on patients with clinically relevant vasospasm after aSAH who underwent endovascular vasospasm treatment and aims to analyze patients’ individual risk factors, intensity, and extent of cerebral vasospasm associated with poor functional outcomes after aSAH.
MATERIALS AND METHODS: We conducted a retrospective cohort study of consecutive patients with aSAH admitted at a tertiary stroke center between January 2016 and December 2022. Patients with medically refractory cerebral vasospasm necessitating at least 1 endovascular intervention were analyzed. Primary end point was defined as functional outcome defined as modified Rankin Scale (mRS) scores after 6 months. Secondary end point was the occurrence of cerebral infarctions following cerebral vasospasm.
RESULTS: Overall, 138 patients received endovascular treatment due to cerebral vasospasm, including 322 treatments, with 78 patients receiving more than 1 endovascular treatment. In 65.2% (90) of patients, cerebral vasospasm developed in both hemispheres; in 16.7% (23), cerebral vasospasm occurred involving the posterior circulation; and in 10.1% (14), percutaneous transluminal angioplasty was performed. Multivariable logistic regression analysis showed an association of higher age (adjusted odds ratio [aOR], 1.05, 95% CI: 1.0–1.1), higher Hunt and Hess grades (aOR, 2.12, 95% CI: 1.38–3.24), the occurrence of rebleeding (aOR, 4.97, 95% CI: 1.0–24.65), and bihemispheric vasospasm (aOR, 4.05, 95% CI: 1.4–11.72) with unfavorable outcome (mRS 3–6). Further analysis showed that higher age (aOR, 1.07, 95% CI: 1.03–1.13) was associated with an increased risk of developing vasospasm-associated infarctions.
CONCLUSIONS: Our results indicate an association between bihemispheric cerebral vasospasm and poor functional outcomes after aSAH. This finding supports a more aggressive treatment strategy in patients developing bihemispheric vasospasm to prevent unfavorable disease courses.
ABBREVIATIONS:
- aSAH
- aneurysmal subarachnoid hemorrhage
- DCI
- delayed cerebral ischemia
- EVD
- external ventricular drainage
- EVT
- endovascular treatment
- ICH
- intracerebral hemorrhage
- IQR
- interquartile range
- mRS
- modified Rankin Scale
- PTA
- percutaneous transluminal angioplasty
- SD
- standard deviation
- TCD
- transcranial Doppler
SUMMARY
PREVIOUS LITERATURE:
Cerebral vasospasms after aSAH are strongly associated with poor functional outcomes. To prevent vasospasm-associated brain injuries, endovascular treatment options can be considered to dilate intracranial arteries, ranging from intra-arterial administration of spasmolytic drugs to transluminal balloon angioplasty. So far, little is known about procedural factors and vasospasm-related characteristics on long-term outcomes.
KEY FINDINGS:
This study represents a large collective of patients with SAH who received endovascular treatment due to cerebral vasospasm after aSAH. Our results present a strong association between bihemispheric vasospasm and poor functional outcomes after 6 months, while prolonged cerebral vasospasm with repeated endovascular treatment was not associated with poor functional outcomes.
KNOWLEDGE ADVANCEMENT:
Since bihemispheric vasospasm is associated with poor functional outcomes, our results support a more aggressive treatment strategy in this subgroup of patients to prevent unfavorable disease courses.
The occurrence of cerebral vasospasm is a strong predictor of poor outcomes following aneurysmal subarachnoid hemorrhage (aSAH).1 Therefore, it is crucial to detect and treat symptomatic and relevant vasospasm early to prevent vasospasm-related infarctions and reduce morbidity and mortality.2 When conservative treatment options for cerebral vasospasm, such as orally administered nimodipine and induced hypertension, are exhausted, endovascular treatment (EVT) can be considered to dilate spastic arteries.3 Intra-arterial administration of spasmolytic drugs or transluminal balloon angioplasty are common endovascular techniques to treat affected intracranial arteries. Additionally, emerging EVT options have been recently described, such as the use of stent retrievers or remodeling stents.4,5 While the intra-arterial administration of spasmolytic drugs has a potential effect on the entire vasculature, mechanical angioplasty of spastic vessel segments might offer greater durability.6 However, angioplasty is also associated with an increased risk of vessel rupture, which can lead to devastating outcomes. The variability in vasospasm treatment, the definition of relevant vasospasm, and different endovascular techniques described in former studies make it difficult to evaluate its effectiveness. Subsequently, because of the lack of data and randomized trials, no commonly accepted treatment guidelines for severe or recurring vasospasm exist.7 While the effectiveness of EVT options is still under discussion, it is also important to understand which patient and imaging-specific risk factors are associated with poor functional outcomes.8,9
The aims of this retrospective analysis are 1) to determine risk factors associated with poor functional outcomes after aSAH and 2) to investigate the influence of onset, duration, and distribution of cerebral vasospasm on functional outcomes. We hypothesized that patients who develop cerebral vasospasm after aSAH are more likely to present with an unfavorable outcome at follow-up. Further, we hypothesized that bihemispheric involvement is an individual risk factor for poor functional outcomes in patients who develop medically refractory vasospasm.
MATERIALS AND METHODS
In this single-center, retrospective study, we included all patients who were treated at our tertiary stroke center with aSAH between January 2016 and December 2022. Inclusion criteria were 1) acute SAH due to a ruptured intracranial aneurysm, 2) medically refractory cerebral vasospasm (= symptomatic vasospasm while under best medical treatment, see below) necessitating at least 1 endovascular intervention, and 3) age >18 years. Exclusion criteria were 1) SAH due to other causes or without evidence of ruptured aneurysm and 2) missing clinical or follow-up data (Supplemental Data).
The study was approved by the local ethics committee (Ärztekammer Hamburg). All study protocols and procedures were conducted in accordance with the Declaration of Helsinki. Patient consent was waived because of the retrospective nature of the study. All data were deidentified and anonymous. For reporting, we used the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist (Supplemental Data).10
Patient Management
SAH was diagnosed by using CT, MRI, or lumbar puncture. Following the SAH diagnosis, a DSA was conducted to confirm the presence of intracranial aneurysms as a cause of SAH. The decision regarding the treatment technique (microsurgical clipping versus EVT) was made by an interdisciplinary neurovascular team consisting of at least 1 neurosurgeon and 1 neuroradiologist and was based on the aneurysm location, configuration, and individual patient characteristics. Depending on the decision made by the interdisciplinary neurovascular team, the aneurysms were treated either by microsurgical clipping or EVT. An external ventricular drainage (EVD) was placed in patients with extensive SAH or imminent hydrocephalus. Serum electrolytes, glucose, renal, and cardiopulmonary functions were checked daily and corrected if necessary.
Diagnosis and Management of Cerebral Vasospasm
Following aneurysm treatment, patients were closely monitored in either the neurosurgical or neurologic intensive care unit. To prevent clinically relevant vasospasm, patients received nimodipine from the day of admission either orally (60 mg/4h) or intravenously (1–2 mg/h). A target value of >80 mm Hg mean arterial pressure was aimed for. Daily clinical examinations were conducted to detect new neurologic deficits, and transcranial Doppler (TCD) measurements were assessed routinely to screen for cerebral vasospasm, as recommended by current guidelines.3 Elevated TCD measurements were defined as a mean velocity >140 cm/s or doubling of the measurements within 24 hours. Patients with new clinical deficits or elevated TCD velocities received additional imaging studies, such as CT including CT angiography and CT perfusion, to confirm cerebral vasospasm and rule out other complications. CT angiography and perfusion images were visually assessed by an interventional neuroradiologist to detect focal or diffuse, newly demarcated narrowing of the vessel caliber and/or focal perfusion deficit. CT perfusion was assessed since it offers high sensitivity and specificity to detect cerebral vasospasm and alterations to the microcirculation that cannot be detected with CT angiography.11 If a new neurologic deficit or a decline of consciousness was detected, which could not be explained by other causes, such as hydrocephalus, re-bleeding, infections, etc, patients were placed on hyperdynamic therapy, ie, induced hypervolemia and hypertension were implemented. The volume therapy was primarily administered with isotonic fluids. The maintenance of a sufficiently high perfusion pressure (ie, a cerebral perfusion pressure of 60–70 mm Hg when measuring intracranial pressure or a mean arterial pressure >80 mm Hg in the absence of intracranial pressure measurement) took priority over the administration of nimodipine. The decision to reduce nimodipine therapy in the context of increasing catecholamine doses was made individually in each intensive care unit, with an evaluation recommended when norepinephrine exceeded 20 μg/min.
Endovascular Interventions
DSA was performed in conscious patients with persistent or worsening neurologic deficits despite hyperdynamic therapy and evidence of pronounced vessel narrowing and/or perfusion deficits in imaging studies that were suspicious for cerebral vasospasm. Unconscious patients underwent DSA in cases of elevated TCD profiles and evidence of pronounced vessel narrowing and/or perfusion deficits in imaging studies.
If relevant vasospasm was confirmed in DSA (which was defined as 1) considerable vessel narrowing in DSA that was consistent with clinical deterioration, defined as the occurrence of focal neurologic deficit or a decline of 2 points or more on the Glasgow Coma Scale,12 which could not explained by other causes or 2) considerable vessel narrowing in DSA in combination with a new perfusion deficit in CTP in the affected vessel territory), a catheter was positioned in the middle cervical internal carotid artery of the affected side or in the middle vertebral artery, and nimodipine was continuously injected over 20 minutes at a standard dosage of 2 mg (in rare cases also 1 mg or 3 mg) per vessel with a final concentration of 0.04 mg/mL and an infusion rate of 2.5 mL/min.
In selective cases with severe and recurrent vasospasm of the proximal intracranial arteries, percutaneous transluminal angioplasty (PTA) via balloon or temporary stent deployment of the affected vessel segment was performed at the discretion of the interventional neuroradiology attending physicians. Patients who received PTA due to vasospasm received periprocedural intravenous heparin and acetylsalicylic acid.
EVT was available 24 hours a day and repeated in cases of recurrent neurologic deficits, increasing TCD velocities, or persisting perfusion deficits. There was no limit to the number of EVTs.
Imaging Analysis
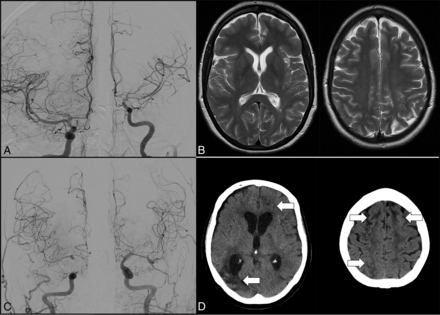
Each case was reviewed retrospectively by an experienced neuroradiologist (>10 years of experience) in a blinded manner to confirm the existence of cerebral vasospasm and was cross-referenced with the operative reports. Vasospasms were defined as a vessel narrowing of at least 25%, which was assessed visually by the rater. The admission DSA was used as a reference when evaluating vasospasm on follow-up DSA. If there was a discrepancy between the retrospective review and the operative reports, a second practitioner would examine the angiogram, and the discrepancies would be resolved by consensus. An example is given in Fig 1.
DSA (A) showing pronounced vasospasms of the left-sided internal carotid artery, M1, and A1 segment in a patient who received intra-arterial nimodipine for 5 consecutive days. Mild vasospasm is also visible in the right-sided A1 segment but was not treated since no symptoms were reported for the right hemisphere. No infarction was detected on MRI at discharge (B) and the patient presented with an mRS of 0 after 6 months. DSA (C) showing bihemispheric vasospasm in the bilateral middle cerebral artery and anterior cerebral artery territories in a patient who received intra-arterial nimodipine for 2 consecutive days. New infarctions were detected in the anterior borderzones and right middle cerebral artery territory (white arrows) on CT scan at discharge (D) and the patient presented with an mRS of 4 after 6 months.
Assessed Variables and Study End Points
Patients’ characteristics were extracted from the digital hospital records. CT images were reviewed by 2 neuroradiologists (consensus rating), blinded to the patients’ characteristics and outcomes, to assess the modified Fisher scale as well as the incidence of intracerebral hemorrhages. Additionally, CT or MR imaging after EVT or neurosurgical treatment and at the time of patients’ discharge were reviewed to detect treatment- and vasospasm-related ischemic lesions.
The primary end point was defined as a functional outcome assessed with the modified Rankin Scale (mRS) after 6 months or, if not available, at the last available follow-up. The secondary end point was the incidence of cerebral infarctions due to cerebral vasospasm.
Statistics
SPSS Statistics 27.0 (IBM) was used for statistical analysis. Data were tested for normality and homogeneity of variance by using histogram plots and the Shapiro-Wilk test. Descriptive statistics are presented as frequencies and proportions (percentages [%]) for categoric variables and compared with Fisher exact test, mean (standard deviation [SD]) for continuous normally distributed variables and compared with the unpaired t test, and medians (interquartile range [IQR]) for non-normal continuous variables and compared with the Mann-Whitney U test, respectively.
For the multivariable logistic regression analyses, only patients who received endovascular vasospasm treatment were included. Clinical outcome was dichotomized into favorable (mRS ≤2) and poor outcomes (mRS ≥3). Multivariable logistic regression analyses to assess risk factors associated with unfavorable outcomes and vasospasm-related infarctions were performed. Variable selection was based on available literature or clinical relevance and is listed in Fig 2. A P value <.05 was considered statistically significant.
Multivariable logistic regression analysis of clinical and procedural variables with (A) follow-up mRS and (B) new infarction at discharge. Only patients who received endovascular vasospasm treatment were included in the analysis. Dots represent the OR and whiskers represent 95% CI.
RESULTS
Patient Demographics
During the 6-year enrollment period, 367 patients with aSAH were treated at our tertiary stroke center. One hundred thirty-eight patients fulfilled the inclusion criteria and received DSA to confirm vasospasm and to initiate EVT. A detailed overview of our study population is given in Supplemental Data. Patients who received endovascular therapy due to medically refractory vasospasm had a higher modified Fisher and Hunt and Hess grade at admission (P = .005 and P = .026). Besides, these patients had a significantly higher rate of ICH at admission and infarctions at discharge (both P < .001). An EVD was more frequently required (P = .036), and longer hospitalization rates (P < .001) and a higher likelihood to experience a poor functional outcome at follow-up (P = .021) were observed in this group.
Endovascular Interventions
A total of 138 patients received EVT due to cerebral vasospasm. Overall, 322 treatments were performed, with 78 patients receiving EVT more than once. The median number of treatments per patient was 2 (IQR 1–3), ranging from a minimum of 1 to a maximum of 11 treatments. Ninety (65.2%) patients developed symptomatic vasospasm in both hemispheres, and 23 (16.7%) patients demonstrated involvement of the posterior circulation. In 14 patients (10.1%; 18/322), PTA was performed. The median time between ictus and the first EVT was 8 days (IQR 5.75–10.25 days) and 11 days (IQR 8–14 days) between ictus and last EVT. The median time between first and last EVT treatment was 2 days (IQR 1–5 days).
Complications
Periprocedural complications were reported in 1.5% (5/322) of patients. In 3 procedures, DSA-related thromboembolic events were reported, in 1 procedure a dissection of the internal carotid artery was reported, and 1 procedure had to be aborted because of a substantial drop in the systemic blood pressure following intra-arterial nimodipine infusion.
Logistic Regression Analyses
Multivariable logistic regression analysis was performed to determine risk factors that are associated with poor functional outcomes and vasospasm-associated infarctions. Only patients who received EVT due to relevant vasospasm were included in this analysis. Two patients had to be excluded because follow-up mRS data was missing.
Patients were divided into 2 groups depending on their functional outcome at follow-up. Favorable outcome (mRS ≤2) was observed in 67 (49.3%) patients, and unfavorable outcome (mRS >2) was observed in 69 (50.7%) patients. Higher age, a higher Hunt and Hess grade, the occurrence of rebleeding, and bihemispheric vasospasm increased the risk for unfavorable functional outcomes at follow-up.
A second multivariable logistic regression analysis was performed to determine risk factors associated with vasospasm-associated infarctions. Only higher age was associated with an increased risk of developing vasospasm-associated infarctions. Bihemispheric vasospasm closely missed statistical significance (P = .053). A detailed overview of the performed multivariable logistic regression analyses is given in Fig 2.
DISCUSSION
This retrospective study investigates risk factors associated with poor functional outcomes after aSAH. The study revealed several main findings: 1) Patients who developed relevant cerebral vasospasm and received EVT for vasospasm treatment had higher rates of new infarctions at discharge and were more likely to present with unfavorable functional outcomes at follow-up, 2) in patients who received EVT due to cerebral vasospasm, higher age, a higher Hunt and Hess grade, the occurrence of aneurysmal re-bleeding and bihemispheric vasospasm were associated with poor functional outcomes, and 3) prolonged cerebral vasospasm, a higher number of endovascular treatments or the necessity of PTA did not show an increased risk for a poor functional outcomes.
The pathophysiology of neurologic deterioration and development of cerebral infarction after aSAH, commonly referred to as delayed cerebral ischemia (DCI), has expanded beyond cerebral vasospasm. This includes cortical spreading depolarizations, neurovascular decoupling, and disturbed microcirculation.13,14 It is important to note that DCI is not solely caused by cerebral vasospasm. However, the occurrence of cerebral vasospasm remains a strong predictor of poor outcomes and the development of cerebral infarctions.1 Our results support previous findings since a higher rate of unfavorable outcomes and new infarctions at discharge were found in patients who developed relevant cerebral vasospasm and received EVT. Although almost two-thirds of patients without relevant vasospasm presented with favorable outcomes (ie, mRS ≤2) at follow-up, only one-half of patients who developed relevant vasospasm presented with favorable outcomes at follow-up. This highlights the ongoing challenge that despite advances in the detection and treatment of cerebral vasospasm, these patients are still vulnerable to permanent disabilities.
To prevent extended brain damage and unfavorable outcomes after aSAH, endovascular therapies are commonly applied as a final option for treating cerebral vasospasm when patients are at risk of developing DCI or cerebral infarction despite receiving the best medical treatment. The effect of these options on the patients’ clinical outcomes is still a topic of critical debate, and complications can occur because of the invasive nature of these procedures.3,8 Certain clinical factors, such as higher Hunt and Hess scores and modified Fisher grading, the presence of intracerebral hemorrhage, and acute hydrocephalus, have been reported to be associated with unfavorable outcomes.15,16 However, little is known about procedural factors and vasospasm-related characteristics that may be associated with poor functional outcomes. Our results suggest that the extent, ie, bihemispheric involvement, but not the recurrence or duration of cerebral vasospasm, is a strong risk factor for poor outcomes. Early onset of vasospasm or the number of endovascular treatments were not associated with an increased risk for poor outcomes. One possible reason for the association between bihemispheric vasospasm and poor outcomes could be the reduced collateral flow through the circle of Willis and communicating arteries. If collateral flow is restricted, the effectiveness of autoregulatory mechanisms, which are already impaired in patients with SAH, or hypertensive therapy may be less effective in compensating for the ischemic state in the affected territories.17 Therefore, it seems comprehensible that a higher number of vasospastic vessels leads to a stronger decrease in brain perfusion, particularly in the watershed areas, and eventually to higher rates of neurologic deterioration and vasospasm-associated infarctions.
Prolonged duration or early onset of vasospasm were not associated with unfavorable outcomes or vasospasm-associated infarctions in our cohort. Schmidt et al18 also reported no significant differences in functional outcomes between patients who developed early (before day 7) or late DCI. However, unlike our results, Schmidt et al reported a higher infarct load in patients with early onset of DCI but did not differentiate between patients who received endovascular treatment and those who did not. We could not find an association between early onset of vasospasm and incidence of new infarction. Also, we did not find an association between the number of endovascular treatments and the incidence of new infarction. This is surprising since endovascular procedures alone can lead to new ischemic lesions.19 Only higher age was a significant risk factor for the development of new infarctions in patients with relevant cerebral vasospasm, while bihemispheric vasospasm showed a trend toward statistical significance (P = .053). Of note, because of their poor clinical status, most patients with vasospasm received a CT scan before discharge, and less than one-third received an MRI. Consequently, smaller infarcts might have been missed, which is a limitation of this study.
We could not find an association between vasospasm in the posterior circulation and poor functional outcomes at follow-up. This is contrary to the results of Cole et al,20 who have shown that patients with vasospasm of the basilar artery were more likely to present with poor outcomes after 6 months. A possible cause of the differing results could be that we also included vasospasms in the posterior cerebral artery and did not exclusively examine isolated basilar artery vasospasms. Further studies are necessary in this regard, and they should also consider collateral circulation via the posterior communicating artery in their analysis.
Recent studies have investigated the effect of repeated endovascular treatments in patients with aSAH and found no harmful effects, while the complication rate was low.21⇓⇓-24 Repeated endovascular treatments, especially the intra-arterial administration of calcium channel blockers, are often necessary because of their time-limited vasodilatory effect.25 Our results support these recent findings since the number of intra-arterial treatments was not associated with poor functional outcomes in our study cohort. Further, our overall complication rate of 1.6% is very similar to previous studies that investigated the feasibility and effectiveness of repeated endovascular vasospasm treatment, ranging from 1.5%–3%.21⇓-23,26 Thromboembolic events were the most common complications in our cohort, with 2 out of 3 occurring during PTA of spastic brain arteries via temporary stent deployment. Overall, PTA was performed in 18/322 (5.6%) procedures, which is less frequent compared with previous studies that performed PTA in 13.8 to 20.5% of all cases.22,26
There are several limitations of this study. First, the present study contains all the limitations that come along with a retrospective study design, including incomplete data (eg, premorbid mRS, etc) and a potential selection bias. Patients who were endovascularly treated due to vasospasm also presented more severely at admission, which could have led to a more escalating treatment strategy in this group, including repeated EVT compared with patients with lower Hunt and Hess scores at admission. Also, it is challenging to distinguish between prognostic factors and possible treatment effects of endovascular procedures, as randomized trials focusing on the impact of such therapies are still missing. There is no uniform definition of cerebral vasospasm, and treatment decisions, especially regarding EVT options, can differ between hospitals. Therefore, it is difficult to generalize our results and compare them with previous studies. Last, we did not perform CT angiography routinely or perform perfusion imaging in conscious patients; rather, we used neurologic and TCD ultrasound monitoring to triage patients for further imaging studies. A similar algorithm was proposed by the 2023 guideline from the American Heart Association/American Stroke Association.3
CONCLUSIONS
While our results confirm the findings of former studies that reported that higher Hunt and Hess scores, higher modified Fisher grading, the presence of intracerebral hemorrhage, and acute hydrocephalus are associated with poor functional outcomes, we also found a compelling relationship between the extent of vasospasm and poor functional outcomes after aSAH. With further advancements and new endovascular techniques to treat cerebral vasospasm, such as balloon angioplasty, the use of stent retrievers, or remodeling stents, our results could support a more aggressive treatment strategy in patients with bihemispheric vasospasm and impaired cerebral autoregulation to prevent unfavorable disease courses. Finally, large randomized controlled studies are needed to demonstrate the benefit of endovascular treatments in a selected patient cohort.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received June 11, 2024.
- Accepted after revision August 30, 2024.
- © 2025 by American Journal of Neuroradiology