Graphical Abstract
Abstract
BACKGROUND AND PURPOSE: CNS embryonal tumor with pleomorphic adenoma gene-like 1 (PLAGL1)/pleomorphic adenoma gene-like 2 (PLAGL2) amplification (ET, PLAGL) is a newly identified, highly malignant pediatric tumor. Systematic MRI descriptions of ET, PLAGL are currently lacking.
MATERIALS AND METHODS: MRI data from 19 treatment-naïve patients with confirmed ET, PLAGL were analyzed. Evaluation focused on anatomic involvement, tumor localization, MRI signal characteristics, DWI behavior, and the presence of necrosis and hemorrhage. Descriptive statistics (median, interquartile range, percentage) were assessed.
RESULTS: Ten patients had PLAGL1 and nine had PLAGL2 amplifications. The solid components of the tumors were often multinodular with heterogeneous enhancement (mild to intermediate in 47% and intermediate to strong in 47% of cases). Nonsolid components included cysts in 47% and necrosis in 84% of the cases. The tumors showed heterogeneous T2WI hyper- and isointensity (74%), relatively little diffusion restriction (ADC values less than contralateral normal-appearing WM in 36% of cases with available DWI), and tendencies toward hemorrhage/calcification (42%). No reliable distinction was found between PLAGL1- and PLAGL2-amplified tumors or compared with other embryonal CNS tumors.
CONCLUSIONS: The study contributes to understanding the imaging characteristics of ET, PLAGL. It underscores the need for collaboration in studying rare pediatric tumors and advocates the use of harmonized imaging protocols for better characterization.
ABBREVIATIONS:
- ATRT
- atypical teratoid/rhabdoid tumor
- ETMR
- embryonal tumor with multilayered rosettes
- ET, PLAGL
- CNS embryonal tumor with PLAGL amplification
- EVD
- external ventricular drain
- IQR
- interquartile range
- PLAGL1
- pleomorphic adenoma gene-like 1
- PLAGL2
- pleomorphic adenoma gene-like 2
- pCASL
- pseudocontinuous arterial spin-labeling
- WHO
- World Health Organization
SUMMARY
PREVIOUS LITERATURE:
CNS embryonal tumor with PLAGL amplification, ET, PLAGL, is a recently defined pediatric tumor characterized by a specific methylation profile and molecular features. A comprehensive re-analysis of tumor tissue of 31 patients initially diagnosed with medulloblastomas, other embryonal tumors, or high-grade gliomas led to the characterization of this distinct tumor type, which is not yet included in the 2021 edition of the WHO classification of CNS tumors. So far, a radiologic description in a larger cohort has been lacking. MRI data from 19 patients were collected from 16 international centers, providing imaging findings of this new tumor.
KEY FINDINGS:
MRI revealed heterogeneous T2WI hyper-/isointensity, like other rare embryonal tumors. However, diffusion restriction was less pronounced. The tumors often contained cystic and/or necrotic areas, showed heterogeneous enhancement, and occasionally presented with hemorrhage and/or calcifications. Only one tumor showed a metastasis at diagnosis.
KNOWLEDGE ADVANCEMENT:
In our cohort, ET, PLAGL could not be clearly distinguished from other embryonal tumors, and no differences were observed between PLAGL1- and PLAGL2-amplified tumors. This study contributes to the imaging characterization of emerging tumor entities within the increasingly detailed tumor classification. It underscores the need for collaboration among centers to enhance our understanding of new, rare tumors.
The molecular and histologic diversity of CNS tumors is increasingly recognized as reflected in the recent 2021 World Health Organization (WHO) classification of CNS tumors, which integrates histology, immunohistochemistry, and molecular features to arrive at a final integrated diagnosis.1 The primary aim of this refined tumor classification is to improve diagnostic and prognostic accuracy, tailor treatment strategies more effectively, and ultimately pave the way for the development of targeted therapies.
Recent advances in DNA methylation profiling, copy number analysis, and DNA/RNA sequencing have led to major refinement in the classification of CNS tumors, including the identification of new tumor types and subtypes.2⇓–4 An example of a new type is the CNS embryonal tumor with PLAGL amplification (ET, PLAGL), a rare type of embryonal tumor mainly seen in children and adolescents, which was first described in 2023 and is not yet included in the WHO classification of CNS tumors.4 Within the category of embryonal tumors, the 2021 WHO classification of CNS tumors distinguishes the more common medulloblastoma subgroups from other, rare embryonal tumors such as the atypical teratoid/rhabdoid tumor (ATRT), embryonal tumor with multilayered rosettes (ETMR), and embryonal tumor not otherwise specified, among which ET, PLAGL has recently been reported as a distinct tumor type. Alterations in the PLAG1 gene are linked to the overexpression of transcriptional factors, playing a crucial role in the development of various tumors including pleomorphic adenomas of the salivary gland, lipoblastomas, hepatoblastomas, and some leukemias.5 Of the three PLAG family genes (pleomorphic adenoma gene 1 [PLAG1], pleomorphic adenoma gene-like 1 [PLAGL1], and pleomorphic adenoma gene-like 2 [PLAGL2]), PLAGL1 and PLAGL2 are specifically linked to CNS embryonal tumors through gene amplification and resultant overexpression of PLAGL1 or PLAGL2. This amplification leads to alterations in associated downstream genes such as imprinted genes and/or potential drug targets.4 We recently re-analyzed tumor samples from 31 patients whose tumors were initially either not classifiable or diagnosed as medulloblastoma, other embryonal tumors, sarcoma, or high-grade glioma and identified ET, PLAGL as a distinct pediatric tumor category. Amplifications of PLAGL1 were primarily found in school-aged children and adolescents (median age, 10.5 years), while PLAGL2 amplifications were more common in younger children (median age, 2 years).4
Currently, there is limited knowledge regarding the outcomes of patients with ET, PLAGL. This uncertainty is compounded by the different treatment strategies used in patients so far.4 Some received radiation therapy (local or as a craniospinal irradiation), others were treated with high-dose chemotherapy, and some underwent both. In the study by Keck et al,4 a difference in overall survival rates was observed between patients with PLAGL1-amplified tumors and those with PLAGL2-amplified tumors (with 5-year overall survival rates of 66% and 25%, respectively) with female patients demonstrating a marginally better outcome, though these results were not statistically significant. Additionally, PLAGL1 amplifications were more commonly found in female patients compared with PLAGL2 amplifications.4
The updated CNS tumor classification necessitates a revised radiologic characterization of both reclassified and newly identified tumor entities to facilitate an accurate presurgical diagnosis, growth patterns, and clinical characteristics. The objective of our study was, therefore, to characterize ET, PLAGL radiologically using conventional MRI data of 19 patients. The hypothesis was that the imaging characteristics of ET, PLAGL would resemble those of other embryonal tumors and that differences between PLAGL1- and PLAGL2-amplified tumors would be observable. The MRI data for this rare tumor were collected from multiple international centers and analyzed by two experienced pediatric neuroradiologists through consensus, applying established conventional imaging criteria.6 Results are presented as descriptive summaries along with illustrations of typical imaging findings.
MATERIALS AND METHODS
Patients
Pseudonymized, pretreatment imaging data of 19 patients with proved ET, PLAGL were provided for central review either by the respective local center or by the national radiology reference center according to the available consent and local ethics vote. Most (n = 15) of these patients were part of the initial study on these tumors conducted by Keck et al.4 Additionally, four patients diagnosed or re-classified as having ET, PLAGL subsequently were included in the present cohort.
MRI Analysis
The MRI data were jointly evaluated in online meetings by two pediatric neuroradiologists (B.B. with 19 years and A.T. with 14 years of experience). They reached consensus decisions on pseudonymized images regarding the following imaging characteristics: 1) anatomic structures involved; 2) cortical, supratentorial WM, and deep GM involvement; 3) the degree of perifocal edema, categorized as none, maximum of 3 mm, <2 cm, or >2 cm; 4) degree of hydrocephalus, categorized as none, mild with ventricular dilation, moderate with ventricular dilation and periventricular edema, or severe with additional sulcal effacement; 5) infiltration of or proximity to the ventricular system (without normal tissue between the tumor and ventricular wall); 6) volume calculated as (craniocaudal × transverse × anterior-posterior diameter)/ 2 (an approximation of the spherical model: [4/3] × π × (craniocaudal/2 × transverse/2 × anterior-posterior/2]); 7) T2WI intensity compared with unaffected cortex; 8) T1WI intensity compared with unaffected cortex; 9) FLAIR intensity compared with unaffected cortex; 10) ADC ratios (ROI with lowest intralesional ADCmean /ROI with ADCmean in contralateral normal-appearing WM; the same applies to ADCmin and ADCmax values); 11) the presence of susceptibility indicating calcification and/or hemorrhage on gradient-echo imaging (T2* or SWI), potentially further specified by T1WI and T2WI; 12) the extent of enhancement, categorized in 6 ranges (0%, 0%–25%, 25%–50%, 50%–75%, 75%–100%, or 100% of the solid tumor component); 13) the strength of enhancement compared with the venous sinus (none, predominantly mild to intermediate, predominantly intermediate to strong); 14) skull involvement and/or scalloping; 15) the presence of nonsolid components, such as thin-walled cysts and/or necrotic regions, the latter showing irregular, thick walls; and 16) dissemination of tumor.
Descriptive statistics were used, including median/interquartile range (IQR). All other variables were reported as percentages.
RESULTS
Patients
Ten (53%) patients had PLAGL1 and nine (47%) PLAGL2 amplifications. As previously known, a higher proportion of girls was observed in the PLAGL1 group, constituting 70% of the patients, compared with 33% in the PLAGL2 group. Detailed information regarding the patients’ sex, age, and PLAGL status is provided in the Table.
| Sex (Female/Male) | Age Median, IQR (yr) | |
|---|---|---|
| All | 10/9 (53%/47%) | 3 (1.8–5.9) |
| PLAGL1-amplified: 10 cases (53%) | 7/3 (70%/30%) | 7.3 (3.9–14) |
| PLAGL2- amplified: 9 cases (47%) | 3/6 (33%/66%) | 1.9 (1.3–2.1) |
Patient demographics and PLAGL1/2 status
Imaging
Brain MRI data were available for all patients. Additionally, 12 patients had spinal MRI scans, and one patient also underwent a brain CT. The MRI data sets predominantly consisted of T2WI, T1WI with and without gadolinium contrast, and FLAIR sequences. DWI was available for 16 patients; T2*, for 7; and SWI, for 2 patients. Pseudocontinuous arterial spin-labeling (pCASL) was performed for 1 patient, and MR spectroscopy was performed in 3 patients (single-voxel, with TEs of 35, 135, and 144 ms).
One patient, a 12-month-old girl with a PLAGL2-amplified tumor, had only T1WI sequences without gadolinium contrast available. Another patient, a 25-month-old boy, also with a PLAGL2-amplified tumor, presented with extensive intratumoral hemorrhage that was partially evacuated. An external ventricular drain (EVD) was placed, followed by a repeat MRI. Analyses were conducted on the second MRI, because the tumor was more discernible in this scan.
A separate case involved a male patient imaged at 17 years of age for unspecified cerebellar symptoms. The MRI revealed an ill-defined, non-space-occupying lesion in the right dentate nucleus and surrounding cerebellar white matter, characterized by hyperintensity on T2WI and hypointensity on T1WI. Although a stereotactic biopsy was performed, it yielded inconclusive results. Seven months later a clearly visible tumor (PLAGL1) was detected on MRI, which was subsequently used for further analysis in our study.
Tumor Localization and Imaging Features
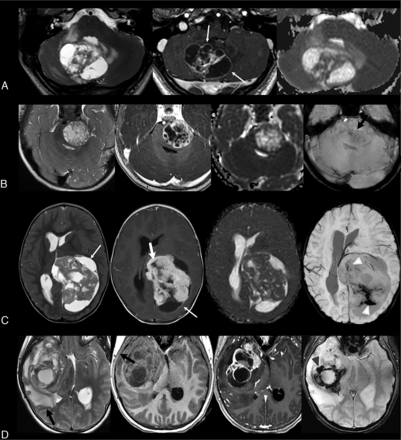
The results are summarized in the Online Supplemental Data, including the distribution according to PLAGL status. Sample images are shown in the Figure and in the Online Supplemental Data.
A, An 18-year-old boy with a PLAGL1-amplified tumor in the right cerebellar hemisphere and the vermis with thin-walled cysts of different sizes (white arrows, second image). The solid parts are sparse, predominantly T2WI isointense, and show heterogeneous, intermediate enhancement. There is no convincing diffusion restriction in the few solid components (ADCmean ratio, 1.51). (from left to right: T2WI, contrast-enhanced T1WI, and ADC). B, A 17-month-old boy with a well-demarcated, PLAGL1-amplified tumor in the left side of the pons, extending into the cerebellopontine angle and the prepontine cistern. The tumor contains numerous small, thick-walled, nonsolid parts, which mainly represent necroses and have no perifocal edema and no clear diffusion restriction (ADCmean ratio, 1.07). The solid components are T2WI iso- to hyperintense and show intermediate-to-strong enhancement. There are very subtle susceptibilities (black arrow on last image) that may arise from hemorrhage or calcification (from left to right: T2WI, contrast-enhanced T1WI, ADC, T2*). C, A 16-month-old girl with a large, multilobulated, PLAGL2-amplified tumor in the left parietal lobe, infiltrating the left lateral ventricle. The nonsolid parts are both thin- and thick-walled (thin and thick white arrows, first and second image) and represent both tumor cysts and necroses. The solid parts are T2WI iso- to hypointense and show intermediate-to-strong enhancement. ADC values are comparable with those in normal tissue (third image; ADCmean ratio, 1.14), and there are susceptibilities in parts of the tumor (arrowheads, fourth image) (from left to right: T2WI, contrast-enhanced T1WI, ADC, SWI). D, A 14-year-old girl with a right temporal, PLAGL1-amplified tumor with strong perifocal edema (black arrow, first image), thinning of the temporal bone (thin black arrow, second image), intermediate-to-strong enhancement, and extensive susceptibilities (gray arrowhead, last image) (from left to right: T2WI, T1WI without and with contrast, SWI).
Eight (42%) tumors were located infratentorially, with five situated in the brainstem with or without involvement of the cerebellar peduncles; and three, in the cerebellar hemispheres with or without affecting the vermis. Ten (53%) were supratentorial, and one was both supra- and infratentorial with three distinct manifestations, located in the right Meckel cave along the trigeminal nerve and left oculomotor nerve area, as well as in the hypothalamus and temporal lobe. Among the supratentorial tumors, two appeared to originate in the lateral and third ventricles. The temporal lobe was implicated in four cases; the insula, in three; the basal ganglia, in two; the thalamus, in two; the parietal lobes, in two; and the frontal and occipital lobes each in one case. Most tumors involved >1 region and anatomic compartment, with the exception of pure brainstem or intraventricular tumors. All other tumors affected the cortex and subcortical/periventricular WM. Perifocal edema was mild or intermediate in 14 (74%) cases and pronounced in two (11%). Hydrocephalus was associated with 11 (58%) tumors (mild in 2, moderate in 4, severe in 5 cases). Five tumors (26%) infiltrated or originated in the ventricular system, while eight (42%) were adjacent to it. The median tumor volume was 50.4 mL (IQR, 14.9–107.6 mL).
The larger solid parts of the tumors were mostly multinodular, ie, consisting of several nodules (eg, Fig C). The tumors were also characterized by many nonsolid components (Fig A, -B) or a combination of both solid and nonsolid features (Fig D). The signal was heterogeneous in most cases, predominantly slightly hyper-/isointense on T2WI and FLAIR and hypo-/isointense on T1WI in relation to cortical GM. The solid components sometimes contained areas of subtle diffusion restriction with an ADCmean ratio of <1 in 5 of the 14 cases (36%; median ADCmean ratio, 0.83; IQR, 0.80–0.94), while the remainder (9/14 cases, 64%) had no diffusion restriction with equal or higher ADC values than the contralateral normal-appearing WM (median ADCmean ratio, 1.16; IQR, 1.11–1.19). ADCmin and ADCmax ratios are shown in the Online Supplemental Data. When SWI or T2* series were available (8 cases, one was after hematoma evacuation and EVD placement), they showed susceptibility in parts of the tumor. Whether it was caused by hemorrhage or calcification could not be reliably determined unless there were unequivocal signs of bleeding with fluid-fluid levels. A 14-year-old girl with a PLAGL1-amplified tumor demonstrated both small calcifications and hemorrhage on CT and SWI (Fig D). In cases in which contrast-enhanced series were available, all tumors showed contrast enhancement, typically presenting in a heterogeneous pattern. The enhancement was predominantly mild to intermediate in 10 (53%) and intermediate to strong in eight (42%) cases, often involving most of the solid tumor component (75%–100% in 9 [47%] patients) or all of it (in 6 [32%] patients).
Skull remodeling was observed in four (21%) patients, including three cases with a supratentorial and 1 with an infratentorial tumor. However, there was no evidence of bony destruction. Notably, not all tumors adjacent to bone showed signs of remodeling. Necrotic regions were present in most tumors (16 cases, 84%), ranging from very small to large, and 47% of the lesions were associated with cysts.
The single data set with pCASL showed elevated CBF. NAA was considerably decreased, and Cho was increased in the three patients who underwent MR spectroscopy. The Cho/NAA ratio was 10.21, 6.81, and 8.31, respectively. No lactate was detected.
One tumor was metastasized both on MRI and in CSF (girl, 8 years 1 month, PLAGL1-amplified) with one lesion affecting the left uncus, hypothalamus, and oculomotor nerve and the other the affecting the right Meckel cave. All other tumors were unifocal, though spinal MRI was available in only 63% of the cases.
DISCUSSION
ET, PLAGL is a recently defined embryonal tumor primarily observed in children and adolescents and has not yet been included in the current 2021 WHO classification of CNS tumors. In this study, we present the MRI characteristics of this new tumor type for the first time and in the largest cohort so far, to raise awareness within the neuroradiology community.
We found no preferential localization with respect to the supra- or infratentorial space, though PLAGL2-amplified tumors were slightly more common in the supratentorial localization, but the low case number does not allow us to draw reliable conclusions. There were no tumors primarily located in the spinal cord. The supratentorial tumors frequently originated in the temporal and insular regions, extending into the deep gray matter, with two appearing to originate from the ventricular system. However, due to their often-substantial size, determining the primary region of origin was challenging. Among the infratentorial tumors, the brainstem was more frequently affected than the cerebellum. At the time of diagnosis, only one tumor was bifocal, indicating dissemination. The remaining tumors were not metastasized, yet only slightly more than one-half of the cases had undergone spine imaging, preventing a definitive exclusion of spinal dissemination.
In our cohort, a common MRI characteristic of both PLAGL1- and PLAGL2-amplified tumors was their often-multinodular structure, accompanied by nonsolid components representing cysts and/or necrotic areas. In some cases, the nonsolid appearance was more prominent, with only a few small nodules or non-nodular, solid components present. All tumors exhibited contrast enhancement that was intermediate to strong in most cases. The T2WI and FLAIR signals were heterogeneous, mainly iso- to mildly hyperintense, while the T1WI signal was primarily hypointense, unless there was calcification and/or hemorrhage present, which could alter the signal. Determining the frequency of hemorrhage and/or calcification was challenging, because T2* and SWI series were often not available. When these modalities were accessible, signal loss was consistently present. In most cases, though, we could not ascertain whether susceptibility were due to blood products or calcifications. ADC values in the solid parts were equal to or higher than those in normal-appearing WM in most cases and lower in a minority of cases. We were not able to identify a discernible pattern that distinguished between PLAGL1- and PLAGL2-amplified tumors. However, this issue is not surprising given the relatively low number of cases.
In our analysis, some ET, PLAGL tumors exhibited MRI characteristics seen in low-grade tumors, such as T2WI hyperintensity and little-or-no diffusion restriction. Like other embryonal tumors, including medulloblastoma, ET, PLAGL tumors are histopathologically defined by primitive, embryonal-like cells with numerous mitoses.4 Generally, such tumors exhibit T2WI hypointensity in at least parts of the lesion and demonstrate diffusion restriction, suggesting high cell density. However, these expected characteristics were rarely observed in our cases. Other embryonal CNS tumors, such as ATRT, ETMR, and CNS neuroblastoma-FOXR2,6⇓⇓–9 also show high proliferation rates and can exhibit T2WI hyperintensity. However, they typically display strong diffusion restriction, which contrasts with most ET, PLAGL tumors in our cohort.
The reasons behind the relatively high T2WI signal and ADC values in these tumors remain unknown. It is well-known that DWI signals are influenced not only by cellularity and the nucleus-cytoplasm ratio, but also by myelin content,10 cell morphology, water compartments, and architectural variances in the respective anatomic region,11 which are not fully captured by standard DWI. Advanced imaging techniques, such as neurite orientation dispersion and density imaging or diffusional kurtosis imaging, might be required to understand these aspects more comprehensively.12,13 In our study, the presence of numerous necrotic regions, detectable both histologically and on MRI, could contribute to the T2WI hyperintensity and relatively high ADC values. This observation underscores the complexity and variability in imaging characteristics of ET, PLAGL, and other embryonal tumors. ADC values are traditionally regarded as a key parameter for differentiating high-grade from low-grade tumors and are particularly useful in distinguishing posterior fossa tumors in children,14 but the increasingly detailed 2021 WHO classification of CNS tumors may challenge some of the conventional imaging principles.
To date, the MRI phenotype of ET, PLAGL has not been systematically described. A recent case report of a 4-year-old girl with a PLAGL1-amplified tumor describes an exophytic pontine tumor with T2WI hypointensity, diffusion restriction, and moderate homogeneous enhancement.15 While these findings seem at odds with our results, the images provided closely match those from cases presented here; we would have characterized the tumor as T2WI isointense to cortical GM with intermediate, slightly heterogeneous enhancement, and no diffusion restriction according to our definition. However, a definitive assessment can obviously only be made with access to the full data set and highlights the need for standardized imaging criteria within the community. Additionally, a recent study by Tauziède-Espariat et al16 discussed two patients with PLAG1 fusions that showed epigenetic, radiologic, and histopathologic similarities to ET, PLAGL. In their study, while a detailed description was not provided, the images displayed similar characteristics such as T2WI iso- to hyperintensity, partially heterogeneous enhancement, signal loss on SWI, and no perifocal edema. However, on the basis of just two cases, it is challenging to conclusively group tumors with PLAG1 fusion and PLAGL1/2 amplification together. Furthermore, as observed in our study, reliably distinguishing these tumors from other, particularly embryonal, tumors remains difficult.
While patient age at diagnosis can be a helpful factor in narrowing down the differential diagnosis in other embryonal tumors, it is less clear in ET, PLAGL. For example, ATRT typically presents in children younger than 2 years of age,17 similar to PLAGL2-amplified tumors (median age, 1.9 years). Patients with PLAGL1-amplified tumors, with a median age of 7.3 years in our cohort, fall into the same age group as those with CNS neuroblastoma-FOXR2 (median age, 5–8 years) and are older than patients with CNS tumors with BCOR-ITD (median age, 4 years) or ETMR (median age, 2.5 years).18 The age of patients with PLAGL2-amplified tumors can also overlap with that of patients with BRAF V600E-mutated astrocytomas, a low-grade glioma that can have similar imaging characteristics with T2WI hyperintensity and occasional diffusion restriction.19 However, in our cohort, PLAGL1-amplified tumors could not be differentiated from PLAGL2-amplified tumors on the basis of imaging. Therefore, they must be summarized as a group with a median age of 3 years, which is similar to that of patients with ETMR. The imaging characteristics of ETMR, however, are distinct, typically characterized by T2WI hyperintensity, weak enhancement, and consistent diffusion restriction.8 Comparative studies on ADC values have been conducted on common pediatric posterior fossa tumors, including medulloblastoma, ependymoma, ATRT, pilocytic astrocytoma, and diffuse midline glioma,20⇓–22 but, to our knowledge, are still lacking for rare embryonal tumors. On the basis of known comparative studies with common pediatric brain tumors, ET, PLAGL appears to have less diffusion restriction than medulloblastomas and ATRT but more than pilocytic astrocytomas and may be comparable with ependymomas.20,22
This study has several limitations. First, the analysis was conducted retrospectively and on a relatively small number of cases, a common challenge when dealing with rare, newly described tumor entities. This feature underscores the importance of close collaboration among centers to collect sufficient cases, especially as tumor classification becomes increasingly detailed and results in inherently low case numbers. Another limitation is the variability and sometimes incompleteness of the MRI data, eg, missing SWI, T2*, or DWI, which hinders the systematic analysis. The adoption of standardized imaging protocols across centers treating patients with pediatric tumors would significantly mitigate this issue.23 Additionally, the limited availability of advanced imaging techniques in our data set represents a considerable constraint.
CONCLUSIONS
We present the most comprehensive MRI data series to date on ET, PLAGL, a rare and recently identified embryonal tumor in children and adolescents. It is not possible to distinguish between PLAGL1- and PLAGL2-amplified tumors on the basis of imaging, nor can ET, PLAGL be differentiated from other embryonal CNS tumors using MRI. Our study contributes to the evolving characterization of new tumor entities, a process increasingly refined by advances in DNA methylation profiling, copy number analysis, and DNA/RNA sequencing. We advocate enhanced collaboration among centers treating these patients, which is crucial for deepening our understanding of emerging tumor entities.
Acknowledgments
Language editing assistance was provided by the large language model ChatGPT.
Footnotes
M.K. Keck and K. von Hoff contributed equally to this work.
This work was funded by the following: B. Bison: German Childhood Cancer Foundation (DKS 2023.10); P.D. Johann: Max-Eder scholarship, German Cancer Aid; T. Perwein: Styrian Children’s Cancer Aid. A. Tietze: German Research Foundation (DFG, SFB295RETUNE). M. Zapotocky: Ministry of Health of the Czech Republic, grant Nr. NU23-08-00460.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received June 23, 2024.
- Accepted after revision September 10, 2024.
- © 2025 by American Journal of Neuroradiology