Abstract
BACKGROUND: Intrameatal vascular loops (IVL) entering the internal auditory meatus and neurovascular contact (NVC) with the vestibulocochlear nerve (cranial nerve [CN] VIII) have been proposed to have a relationship with audiovestibular symptoms.
PURPOSE: This systematic review and meta-analysis aimed to determine whether the presence of IVLs and CN VIII NVC on MRI is associated with tinnitus, sensorineural hearing loss (SNHL), or vertigo and any specific subtypes.
DATA SOURCES: All studies comparing the presence of IVL or CN VIII NVC in ears with these audiovestibular symptoms and controls were identified through MEDLINE, EMBASE, the Web of Science Core Collection, Scopus, and the Cochrane Register of Controlled Trials databases.
STUDY SELECTION: Sixteen studies and 3455 ears (1526 symptomatic ears and 1929 control ears) were included.
DATA ANALYSIS: Meta-analysis was performed using a bivariate random effects model. Pooled ORs were calculated, and heterogeneity was evaluated with the Cochran Q test with statistical significance defined as P < .05.
DATA SYNTHESIS: There was no significant association between the presence of undefined tinnitus or SNHL and IVL (OR, 0.90; 95% CI, 0.47–1.70; OR, 0.67; 95% CI, 0.36–1.25) or CN VIII NVC (OR, 1.15; 95% CI, 0.68–1.95; OR, 0.89; 95% CI, 0.33–2.40). However, the subgroup of sudden onset SNHL was associated with IVL (OR, 1.34; 95% CI, 1.04–1.73) (P = .02). There was no significant difference in the prevalence of IVL (OR, 0.97; 95% CI 0.64–1.48) or CN VIII NVC (OR, 0.99; 95% CI, 0.42–2.32) between ears with undefined vertigo and control ears. However, there was an association between the presence of CN VIII NVC and the specific diagnosis of vestibular paroxysmia (OR, 13.19; 95% CI, 2.09–83.16) (P = .006).
LIMITATIONS: Our meta-analysis is limited by selection bias, the small number of eligible studies, and moderate heterogeneity.
CONCLUSIONS: IVL or CN VIII NVC on MRI is unrelated to symptoms of undefined tinnitus, SNHL, and vertigo. However, CN VIII NVC is associated with vestibular paroxysmia, while IVL is associated with sudden onset SNHL.
ABBREVIATIONS:
- CN
- cranial nerve
- IAM
- internal auditory meatus
- IVL
- intrameatal vascular loop
- NVC
- neurovascular contact
- SNHL
- sensorineural hearing loss
- SoSNHL
- sudden onset sensorineural hearing loss
Vascular loops are frequently identified within the internal auditory meatus (IAM) on MRI. While this is most commonly due to the anterior inferior cerebellar artery, the posterior inferior cerebellar artery, superior cerebellar artery, or venous structures may also be responsible.1 Authors have speculated that these intrameatal vascular loops (IVLs) may be responsible for tinnitus, sensorineural hearing loss (SNHL), and vertigo.2 It is hypothesized that such audiovestibular symptoms may result from altered neural conduction due to cranial nerve (CN) VIII neurovascular contact (NVC), reduced vascular perfusion of the nerves due to disturbed blood flow, or increased transmission of sound through the CSF of the IAM.1,2 Symptoms due to NVC are considered most common when a vessel contacts the centrally myelinated portion of the CN or the transition zone between central and peripheral myelin.3
Determining an association between the presence of an IVL or CN VIII NVC and audiovestibular clinical presentations would guide the selection of appropriate diagnostic MRI sequences and interpretation of the imaging findings. There are also potential therapeutic implications because vascular decompression surgery has been performed to alleviate such symptoms.4 An association between these vascular variants and audiovestibular clinical presentations remains uncertain, with variable outcomes from previous case-controlled studies and with no contemporary pooling of the statistical outcomes.
This systematic review and meta-analysis aimed to determine whether there is an association between IVLs or CN VIII NVC and specific audiovestibular symptoms.
MATERIALS AND METHODS
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines5 and enrolled in the Prospective Register of Systematic Reviews (PROSPERO), CRD42023447065.
Search Strategy
The search strategy was designed using population; intervention or exposure; comparator/control; outcome; study design (PICOS). “Population” was defined as ears with specific audiovestibular symptoms; “exposure,” as the presence of an IAM vascular loop or CN VIII NVC; “control,” as ears without audiovestibular symptoms; “outcome,” as the presence of an IAM vascular loop or CN VIII NVC relative to the reporting of audiovestibular symptoms; and “study design,” as case-controlled cross-sectional studies. Search terms were adapted after a pilot search to include relevant synonyms (Online Supplemental Data).
Searches were performed in MEDLINE, EMBASE, the Web of Science Core Collection, Scopus, and the Cochrane Register of Controlled Trials databases. The search was performed from database inception onward. The searches were last performed on January 8, 2023. Manual forward and backward searches were performed for all eligible review articles. The 5 most frequently cited journals were hand-searched (2009–2023) (Online Supplemental Data). EndNote Web (https://endnote.com/) was used as a reference manager to identify and collate the literature and allow the manual removal of duplicates.
Selection of Studies
Two independent reviewers (J.E.C. and M.S.T.) applied the piloted screening tool to the titles and abstracts with the following inclusion criteria: a defined patient group with audiovestibular symptoms; analysis using MRI; and reference to a vascular loop/variant or anomaly. Case studies, review articles, foreign language literature, and duplicated studies were excluded. The full text was independently assessed for eligibility by both reviewers according to the PICOS criteria. Studies were included when there were data that could be extracted into a 2 × 2 contingency table comparing the presence of an IVL or CN VIII NVC in ears with and without tinnitus, SNHL, or vertigo. Because vertigo is not lateralized to either ear, patients with vertigo were compared with subjects without vertigo, with the prevalence of an IVL or CN VIII NVC in either ear being recorded. When a symptomatic subtype was defined by specific criteria, then this subtype was analyzed separately.
Reasons for exclusion are listed in the Online Supplemental Data. Discrepancies were resolved by discussion (J.E.C., M.S.T., S.E.J.C.).
Data Extraction
Two reviewers (J.E.C., M.S.T.) independently extracted data regarding the following: 1) study characteristics: authors, year of publication, retrospective versus prospective, case and control group size, IVL classification scale applied, specified audiovestibular symptoms; 2) control group type: asymptomatic contralateral ears, healthy volunteer ears, or ears of patients with an unrelated condition; and 3) demographic and clinical characteristics: age and sex of the case and control groups, and unilateral or bilateral cases. Contingency tables (2 × 2) were constructed comparing the presence of the audiovestibular symptom (criterion standard) with the presence of an IVL or CN VIII NVC on MRI (index test). Details of specific classifications for the assessment of IVL and CN VIII NVC are provided in the Online Supplemental Data. Any discrepancies in the data collection were resolved by revisiting the article in a consensus meeting.
Quality Assessment
The methodologic quality of the eligible studies was evaluated using the Quality Assessment of Diagnostic Accuracy Studies, Version 2 (QUADAS-2; https://www.bristol.ac.uk/population-health-sciences/projects/quadas/quadas-2/) tool by 2 independent reviewers (J.E.C., M.S.T.).6 The signaling questions were tailored specifically to the review (Online Supplemental Data).
Statistical Analysis
Bivariate random effects meta-analysis was performed with R 4.3.1 (package “meta”; http://www.r-project.org/) and R Studio (Version 2023.09.0 + 463; http://rstudio.org/download/desktop) to evaluate the association between audiovestibular symptoms and the presence of an IVL or CN VIII NVC. The results were tabulated with corresponding forest plots. Pooled ORs were calculated with 95% CIs and P values. Heterogeneity was assessed by the Cochran Q test. Further quantification of heterogeneity was provided with τ2 and I2 statistics. Statistical significance was defined as P < .05.
RESULTS
Systematic Review
The screening tool identified 77 potentially eligible articles. After full text review, 15 studies were included. One additional relevant article was identified via citation searching, totaling 16 eligible studies (Fig 1).
Flow chart summary of the systematic literature review process.
Study Characteristics
The demographics of the patients from eligible studies and the number of case (with audiovestibular symptoms) and control ears are documented in Table 1.1,7⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓-21 A total of 1526 symptomatic ears and 1929 control ears were included. The mean ages ranged between 43 and 54.1 years. Study characteristics are demonstrated in the Online Supplemental Data. Eligible studies comprised ears with tinnitus (n = 5), SNHL (n = 7), and vertigo (n = 6). Specific symptomatic subtypes studied were “typewriter tinnitus,” sudden onset sensorineural hearing loss (SoSNHL), and vestibular paroxysmia. The diagnostic criteria for these subtypes are defined in the Online Supplemental Data. Control groups comprised the contralateral ear (n = 8), a healthy asymptomatic patient cohort (n = 5), or a patient cohort with an unrelated condition (eg, trigeminal neuralgia) (n = 3).
Eligible studies and patient demographics
Eleven articles analyzed the relationship of audiovestibular symptoms to IVL, while 13 articles analyzed the association with CN VIII NVC. The most adopted classification regarding the position of the IVL was that proposed by Chavda in McDermott et al.22 A standardized classification for NVC was seldom used, with that established by Gorrie et al11 adopted in 3 articles (Online Supplemental Data).
Quality of Studies
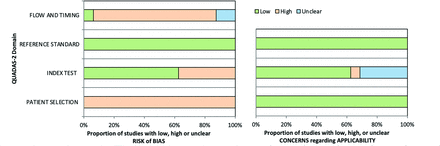
QUADAS-2 evaluation showed high bias within the “patient selection” and “flow and timing” domains. Patient selection always resulted in high bias due to all included studies being case-controlled. High bias was reported for the index test when it was unclear whether readers were blinded to the clinical details. Because the diagnosis of an audiovestibular symptom (criterion standard) always preceded index test evaluation, all studies were low bias in this domain. Only 1/16 studies was deemed low risk with regard to flow and timing because others were either retrospective or included post hoc exclusions (Fig 2).
QUADAS-2 tool. Bar charts quantify studies by risk of bias (A) and for the 16 eligible studies included in the meta-analysis concerns (B).
Relationship between Audiovestibular Symptoms and an IAM Vascular Loop or CN VIII NVC
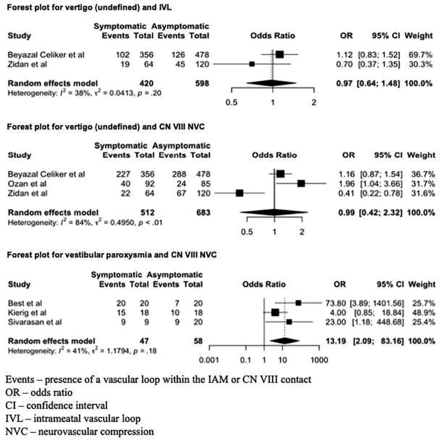
Pooled odds ratios are presented in Table 2. Forest plots are shown in Fig 3.
Continued.
Forest plots for each audiovestibular symptom and corresponding MRI variable (IVL or CN VIII NVC).
Pooled ORs, 95% CIs, and P values for the association between IVL or CN VIII NVC and audiovestibular symptoms
There was no significant association demonstrated between the presence of tinnitus (undefined) and IVL (OR, 0.90; 95% CI, 0.47–1.70) or CN VIII NVC (OR, 1.15; 95% CI, 0.68–1.95) (P > .05). The single study of the typewriter tinnitus subtype showed no significant difference in the prevalence of symptoms in ears with either IVL (P = .73) or NVC (P = .15).7
There was no relationship demonstrated between ears with SNHL (undefined) and the presence of IVL (OR, 0.67; 95% CI, 0.36–1.25) or CN VIII NVC (OR, 0.89; 95% CI, 0.33–2.40) (P > .05). In studies of the SoSNHL subtype alone, there was also no relationship between symptomatic ears and CN VIII NVC (OR, 1.16; 95% CI, 0.61–2.20) (P > .05); however, there was an association with an IVL (OR, 1.34; 95% CI, 1.04–1.73) (P = .02).
There was no significant difference in the prevalence of IVL or CN VIII NVC between ears with vertigo (undefined) and control ears (OR, 0.97; 95% CI, 0.64–1.48 and OR, 0.99; 95% CI, 0.42–2.32) (P > .05). However, there was a strong association between the presence of CN VIII NVC and the specific diagnosis of the vestibular paroxysmia subtype (OR, 13.19; 95% CI, 2.09–83.16) (P = .006). No included studies investigated the association of IVL and vestibular paroxysmia.
Heterogeneity
Considerable heterogeneity was detected in 1 subgroup for articles comparing the presence of CN VIII NVC in patients with vertigo (undefined) (P < .01), with an I2 of 84%. All other analyses demonstrated nonsignificant P values (range, .05–.71). I2 quantification suggested moderate heterogeneity for 5 subgroup analyses and substantial heterogeneity for 2 further subanalyses. The subgroup analysis comparing IVL and SNHL demonstrated an I2 of 0%. A guide for the interpretation of I2 has been included in the Online Supplemental Data.
DISCUSSION
This systematic review and meta-analysis sought to quantify the relationship between IVL or CN VIII NVC and specific audiovestibular symptoms. Pooled data from 16 case-controlled studies demonstrated no statistically significant association between the presence of an IVL or CN VIII NVC and either tinnitus (undefined) or SNHL (undefined), while there was also no relationship between IVL or vertigo (undefined) or CN VIII NVC and vertigo (undefined). However, the symptomatic subtype of SoSNHL demonstrated a significant association with the presence of an IVL, while the symptomatic subtype of vestibular paroxysmia was associated with CN VIII NVC (Fig 3) (Table 2).
Few attempts have been made to formally synthesize the body of literature before now. Chadha and Weiner23 performed a systematic review of 5 articles in 2008, but this comprised studies with heterogeneous and largely noncomparable methodology. Two of the eligible studies concurred with our finding of no significant association between CN VIII NVC and unilateral tinnitus; however, an association was demonstrated between CN VIII NVC and sensorineural hearing loss (P < .05; OR, 1.99).23 In addition, a more recent meta-analysis found no significant association between ipsilateral SoSNHL and NVC, though substantial differences in the eligibility criteria and the interpretation of IVL/NVC grading exist between their work and the present study.24
The results of the current study should serve to inform both radiology and surgical colleagues alike. While dedicated 3D T2-weighted sequences will be required to exclude other pathologies in patients with undefined audiovestibular symptoms, there will not be a requirement for the radiologist to report either CN VIII NVC or IVL. Similarly, our analysis indicates that IVL and CN VIII NVC are unlikely to be an etiology for undefined SNHL, tinnitus, and vertigo and would not benefit from surgical intervention. A previous meta-analysis of 35 studies and 572 patients who underwent microvascular decompression of CN VIII NVC found that only 28% of patients with tinnitus and 32% of those with vertigo reported complete relief of symptoms, with substantial complication rates following treatment.4
The results of the current study demonstrate an association between an IVL and SoSNHL. Identifiable causes of SoSNHL are found in a minority of patients, with a vascular etiology and altered neural perfusion in the context of an IVL a possible pathophysiologic explanation.25 However, of the 5 articles included in the subanalysis, one demonstrated an OR of >1, suggesting low statistical power for this association. Moreover, there may be several confounding factors responsible for the altered vascular supply to the inner ear, with a recent meta-analysis demonstrating increased rates of concomitant diabetes, hypertension, and increased cholesterol in patients with SoSNHL.26
The demonstrated association of CN VIII NVC with vestibular paroxysmia also warrants a consideration of potential pathophysiology. While this study indicates an association rather than causality, it may be postulated that the association of CN VIII NVC with the characteristic short-lived paroxysmal vertigo of vestibular paroxysmia is more likely to be related to direct pulsatile compression of the vulnerable long VIII transition zone resulting in ephaptic discharges.27
Limitations of the current study should be appreciated. First, a body of non-English language literature was not reviewed, possibly missing eligible or noteworthy studies. Second, the low number (range, 2–5) of eligible studies for each analysis and the generally small study cohorts have the potential to increase type II errors. In this regard, some substantial case-controlled studies were not considered eligible because it was not possible to identify the specific audiovestibular symptom experienced. For instance, Makins et al28 studied “auditory symptoms,” and Lei et al29 investigated a cohort with Meniere disease, neither of which showed a significant association with IVLs. Third, case-controlled studies analyzing IVL or CN VIII NVC were not available for all relevant symptom subgroups, including for pulsatile tinnitus. Finally, despite attempts to reduce heterogeneity in our meta-analysis and P values ≥ .05 in 7/8 plots, there remained a moderate-substantial degree of variance with I2 ranging from 0% to 84% for each subanalysis. Explanations are likely multifaceted and comprise clinical heterogeneity between studies and a small number of included articles. Any future studies should aim to collect patients consecutively, with a sample size that allows appropriate power, and to adopt an established grading system for vascular loops and a control group of healthy volunteers.
CONCLUSIONS
This systematic review and meta-analysis evaluated the association between the presence of a vascular loop within the IAM (IVL) or contacting the vestibulocochlear nerve (CN VIII NVC) and audiovestibular symptoms. There was no statistically significant correlation between IVL or CN VIII NVC and tinnitus, SNHL, or vertigo, except for specific subtypes of vestibular paroxysmia and SoSNHL.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received June 14, 2024.
- Accepted after revision August 15, 2024.
- © 2025 by American Journal of Neuroradiology