SUMMARY:
Spinal CSF leaks from dural tears or CSF-venous fistulas are the most common causes of spontaneous intracranial hypotension. Rarely, CSF leaks have also been associated with vascular malformations, which have primarily been discussed in case reports or small series. In this clinical report, we report the clinical features, imaging findings, and treatment of 6 children and adults with CSF leaks associated with vascular malformations in the spine and skull base depicted on CT myelography and cisternography.
ABBREVIATIONS:
- ISSVA
- International Society for the Study of Vascular Anomalies
- SIH
- spontaneous intracranial hypotension
Spontaneous intracranial hypotension (SIH) is most commonly secondary to a spinal CSF leak either from a dural tear or CSF-venous fistula.1 Apart from these spontaneous defects, SIH has also been described with vascular malformations, namely venous and lymphatic malformations, which are either isolated lesions or in patients with syndromes.2⇓⇓⇓⇓-7 Despite the prior reports, understanding these diseases has been hampered by incorrect nomenclature. Regarding SIH, previous publications have inappropriately reported vascular malformations associated with “Chiari I malformation,” when, in fact, the lesions were resulting in SIH.8,9 Vascular malformations are also often mischaracterized despite nomenclature from the International Society for the Study of Vascular Anomalies (ISSVA).10 For example, CSF leaks from underlying hemangiomas and “lymphangiomatosis” have been described,9,11 which now may not be appropriate nomenclature, owing to our greater understanding of the genomics of vascular malformations. Last, myelographic evidence of CSF leaking into a vascular malformation has only been briefly described in some reports. In this clinical report, we present 6 cases of SIH secondary to spinal or skull base CSF leaks from an underlying vascular malformation and discuss the proper terminology, myelographic techniques, and possible therapeutic options.
Case Series
Institutional review board (Kaiser Permanente) approval was granted, which waived the right for informed consent. The study population consisted of patients with SIH associated with a vascular malformation between 2000 and 2024. Inclusion criteria were the following: 1) diagnosis of SIH according to the International Classification of Headache Disorders, Third Edition;12 2) spinal or skull base CSF leak identified on CT myelography or cisternography; and 3) vascular malformation appropriately categorized according to the latest ISSVA classification after multidisciplinary review in the vascular anomalies clinic or by a radiologist experienced with vascular anomalies. Brain MRIs were assessed using the SIH Bern scoring system.13
Six cases of CSF leaks associated with an underlying vascular malformation were identified (Table). There were 4 females and 2 males, and the mean patient age was 20.8 years (range, 7–40 years). Three of the patients were pediatric (younger than 18 years of age). Four of the CSF leaks were in the spine, and 2 were in the skull base. There were 3 patients with CSF leaks with a venous malformation and 3 with a lymphatic malformation, and all 3 patients with a lymphatic malformation had a complex lymphatic anomaly.14 All patients had a paraspinal or skull base vascular malformation. Two of the patients were previously reported by our group and are being included to evaluate this unique cohort holistically.6,7
Case 1
A 23-year-old woman presented with 8 months of positional headaches that prompted an MRI of the brain and spine, which showed severe brain sag, cerebellar tonsillar descent, and a syrinx, yielding the initial diagnosis of “Chiari I deformity.” She was being evaluated by the neurosurgery service for potential suboccipital decompression. Follow-up brain imaging was interpreted by a member of the CSF leak team, and a diagnosis of SIH was made with a Bern score of 6 without dural enhancement (Fig 1). In a review of the spine imaging at 18 months of age, there had been an enhancing epidural lesion in the thoracolumbar spine that was thought to represent an epidural hematoma or a “hemangioma.” Twenty-one years later, she presented with headache and had a spine MRI that showed marked dural ectasia at the T12 level, with a previously undiagnosed paraspinal venous malformation. A right decubitus CT myelogram was obtained with initial scanning of the thoracolumbar spine that showed subarachnoid contrast at the T12 dural ectasia but no leakage. Delayed imaging after 3 minutes and an additional small volume of contrast showed a CSF leak in the right T11–T12 neural foramen, commencing from the thecal sac and extending to portions of the venous malformation near the pleura. A targeted fibrin glue patch was administered, with moderate relief in symptoms to date 3 months after the procedure.
Patient 1. Spinal CSF-venous malformation fistula. A 23-year-old woman had a history of an “epidural hematoma” at 18 months of age. Twenty-one years later she developed postural headaches. A, A sagittal T2-weighted image of the brain shows severe midbrain sag (arrow), along with cerebellar tonsillar descent and a cervical cord syrinx (arrowhead). B, Axial contrast-enhanced fat-suppressed T1-weighted image at 18 months of age shows an enhancing lesion in the T12 epidural space (arrows) that extended into the right paraspinal soft tissues. C, Sagittal STIR obtained as an adult shows a large right paraspinal lesion (arrows) that was consistent with a venous malformation. D, Axial T2-weighted image shows the paraspinal venous malformation (arrowheads) and right dural ectasia at the T12 level (arrow). E, A right decubitus CT myelogram obtained in the coronal plane shows opacification of the right T12 dural ectasia (arrow) but no leakage. A left-sided phlebolith is noted (arrowhead), which is consistent with the underlying paraspinal venous malformation that extended to the left side. Delayed myelographic images 3 minutes later show a right T11–T12 fistula between the thecal sac and the venous malformation (F, arrow), with additional opacification of the venous malformation more laterally near the ribs (G, arrow). H, A fibrin glue patch filling the CSF-venous malformation fistula (arrow) provided moderate relief.
Case 2
A 40-year-old man with a long-standing history of Chiari I deformity presented to our CSF leak program after suspicion of SIH by the clinical team. Brain and spine MRIs showed severe brain sag, syrinx, and a Bern score of 6 without dural enhancement. Review of the medical records indicated that he had a suboccipital decompression at 22 years of age, which did not resolve the brain sag or syrinx. Seven years later, the patient had a T1–T3 decompression for a dorsal epidural lesion that was diagnosed radiologically and at pathology as a hemangioma. In retrospect, this lesion represented a venous malformation (Online Supplemental Data). After he presented to our CSF leak program, a right decubitus CT myelogram was obtained and showed a right T1–T2 CSF-venous fistula, which was successfully treated with targeted fibrin glue patching. Although the CSF-venous fistula did not drain into the venous malformation given its prior resection, it was thought to be related given that it was located at the same thoracic level and the intracranial SIH findings were persistent despite the prior surgery. The follow-up decubitus CT myelogram showed resolution of the CSF-venous fistula.
Case 3
A 33-year-old man with a large left scalp, facial, and neck venous malformation presented with headaches and upper and lower extremity numbness. MR imaging of the brain and neck (Online Supplemental Data) showed an extensive venous malformation along the left temporal scalp, erosion of the calvaria, and contiguity with the brain parenchyma. Imaging also showed severe brain sag, cerebellar tonsillar descent, and a cervical syrinx that was diagnosed as a Chiari I deformity. Scans showed a Bern score of 4 without dural enhancement. A suboccipital decompression was performed that did not resolve the radiologic and clinical findings. Bilateral decubitus myelograms did not show a spinal CSF leak, but a CT cisternogram showed leakage into the venous malformation within the retromandibular fossa. Dural ectasia was also observed along the left temporal lobe with multiple cephaloceles. Targeted sclerotherapy has been performed for the venous malformation, but the skull base leak has not yet been repaired.
Case 4
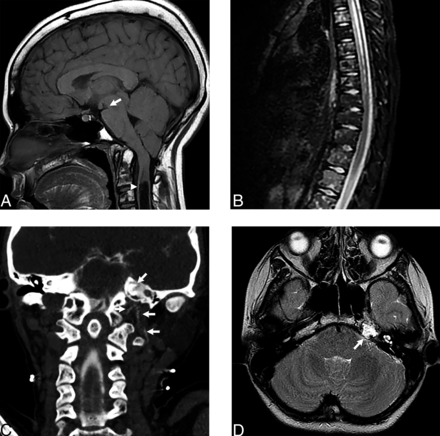
A 13-year-old girl presented with headaches and facial numbness. She was previously diagnosed with a generalized lymphatic anomaly, which was first suspected after she presented with diffuse microcystic lymphatic malformations in the right lower extremity and pelvis, with a resultant bladder fistula that caused chyluria. Multiple hematologic and electrolyte abnormalities were present on laboratory analysis, which were in keeping with a generalized lymphatic anomaly as opposed to Gorham-Stout disease, another complex lymphatic anomaly. Her brain and spine MRI showed brain sag, cerebellar tonsillar descent, and a syrinx, yielding the initial diagnosis of Chiari I deformity (Fig 2). The case was evaluated in the Vascular Anomalies Clinic, and SIH was diagnosed with a Bern score of 4 without dural enhancement. The spine MRI showed diffuse intraosseous lymphatic malformations but no extradural collection or large meningeal diverticula. Bilateral decubitus CT myelograms were performed, which did not reveal a CSF-lymphatic malformation fistula. In the same procedural session, the skull base was imaged and revealed frank CSF leakage from the left petrous apex that extended into the left neck musculature to the C3 level. In retrospect, the CSF leak was observed in the neck on the myelograms, but the leak site was not seen due to incomplete visualization of the skull base. The prior brain MRI was also reviewed and showed a lymphatic malformation in the left petrous apex. Skull base surgery is planned for treatment.
Patient 4. Skull base CSF-lymphatic malformation fistula in a 13-year-old girl who had a history of a generalized lymphatic anomaly for many years and presented with more recent onset of headaches. A, Sagittal T1-weighted image of the brain shows midbrain sag (arrow), along with cerebellar tonsillar descent and a syrinx (arrowhead). B, Sagittal STIR image of the thoracic spine shows diffuse intraosseous lymphatic malformations. Bilateral decubitus CT myelograms did not show a CSF fistula (not shown), but a CT cisternogram of the skull base (C) shows frank leakage from the left petrous apex into the left neck musculature (arrows) that extends to the C3 level. D, Axial T2-weighted image shows a left petrous apex lymphatic malformation (arrow) that was observed in retrospect.
Case 5
A 9-year-old girl with a history of kaposiform lymphangiomatosis, which was diagnosed during infancy after she presented with Kasabach-Merritt phenomenon and diffuse lymphatic malformations throughout the body, complained of headaches for several years that were initially thought to be migraines. Brain MRI was eventually performed, and a diagnosis of SIH was made with a Bern score of 6, including severe brain sag but no dural enhancement. A spine MRI showed a syrinx and diffuse intraosseous lymphatic malformations but no extradural collection or visible meningeal diverticula. A ventral prone CT myelogram was performed that showed rapid CSF accumulation in the right T10–T11 neural foramen and paraspinal soft tissues, which opacified the thoracic duct to the level of the left innominate vein, resembling a lymphangiogram (Fig 3). In retrospect, a paraspinal lymphatic malformation on the prior MRI was visible at the right T10–11 level. Treatment with targeted blood patches provided incomplete relief; therefore, surgical ligation of the CSF lymphatic fistula resolved symptoms after a period of rebound hypertension.
Patient 5. Spinal CSF-lymphatic malformation fistula in a 9-year-old girl with a history of kaposiform lymphangiomatosis who had chronic headaches. Brain and spine MRI demonstrated severe brain sag, syrinx, and intraosseous spinal lymphatic malformations (not shown). Coronal images from a prone CT myelogram show rapid egress of contrast from a right T10–T11 meningeal diverticulum to the paravertebral space (A, arrow). The thoracic duct also opacified to the level of the innominate vein (B and C, arrows), resembling a lymphangiogram procedure. In retrospect, there was a lymphatic malformation identified in the right T10–T11 paravertebral space on MRI (not shown).
Case 6
A 7-year-old girl with Gorham-Stout disease presented with postural headaches. During infancy, she had marked irritability, and MRIs of the brain and spine showed brain sag, cerebellar tonsillar descent, and a syrinx that was initially diagnosed as a Chiari I deformity, for which a suboccipital decompression was performed. The SIH Bern score was 6, and there was no dural enhancement. At 7 years of age, spine MRI showed diffuse destructive intraosseous lymphatic malformations throughout the lumbar spine. A prone CT myelogram showed CSF leakage from the right L4 nerve root into the vertebral body and paraspinal soft-tissue lymphatic malformations (Online Supplemental Data). Targeted blood patches resulted in transient relief. Subsequently, a targeted n-BCA liquid embolic was percutaneously injected via a spinal needle into the fistula, which resulted in marked improvement in symptoms. During the following 15 years, the patient has intermittently had both low- and high-pressure symptoms, which have been treated with intermittent blood patches, lumbar and ventricular peritoneal shunting, and medical therapy.
DISCUSSION
Spinal CSF leaks associated with adjacent vascular malformations are uncommon. In this clinical report, we demonstrated 6 patients with CSF leaks at CT myelography or cisternography directly caused by spinal or skull base vascular malformations.
Similar to routine CSF-venous fistulas, the pathogenesis of CSF-vascular malformation fistulas has not been well-characterized, but invasion of either the dura and/or nerve root sleeve by the malformation may result in the fistulous connection. This outcome is not uncommon for vascular malformations in other parts of the body. For example, patient 4 developed chyluria from a microcystic lymphatic malformation invading the bladder wall. Similarly, patient 1 had an epidural and paraspinal venous malformation at 18 months of age but did not manifest her SIH symptoms until 2 decades later, when a fistulous connection between the thecal sac and the venous malformation was detected. Patient 2 also had an epidural venous malformation and developed a CSF-venous fistula years later. Further studies are needed to explain how these fistulas form; however, a clear observation is that the presence of an epidural or paraspinal vascular malformation is a potential risk factor for a CSF fistula.
Myelographic timing and density are complementary factors for detection of CSF-venous fistulas.15 Similarly, timing may be important for CSF-venous malformation fistulas. In patient 1, the CSF-venous malformation fistula was not visible on the early myelographic phase immediately after contrast administration but was apparent on a delayed scan 3 minutes later. We suggest that multiphase scanning may be helpful in this patient cohort. Analogously, venous malformations can often demonstrate delayed enhancement at MR imaging due to their venous nature.16
In all patients, there was a delay in the diagnosis of SIH. Five of 6 patients were incorrectly diagnosed with a Chiari I deformity, 3 patients had a suboccipital decompression as a result, and 2 patients were being evaluated for a suboccipital decompression before the correct diagnosis was established. Confusion between Chiari 1 and SIH occurs frequently despite criteria differentiating these 2 entities,17 particularly among pediatric patients in whom SIH is less common than in adults. One major distinction between Chiari I deformity and SIH is the mamillopontine distance. In SIH, this distance is often reduced, while in Chiari I, it is typically normal.17 Four of our 6 patients had severe brain sag, which we characterized by an absent mamillopontine distance and could be due to the severity of the hypotension. The presence of a cervical cord syrinx was another imaging feature that also likely contributed to the incorrect diagnosis of Chiari deformity. SIH can rarely result in syrinx in patients without vascular malformations,18 but it is not fully understood why a syrinx was present in all patients in this cohort with vascular malformations. Perhaps, chronic leakage into a vascular malformation results in lower CSF pressure and greater descent of the cerebellar tonsils. In pediatric patients, another theory could be due to reduced posterior fossa calvarial development. The calvaria is still developing in childhood, and this development may lead to more cerebellar tonsillar descent due to the intracranial hypotension. This rationale is the same as the one for why some studies prefer the term Chiari I deformity rather than malformation, because it is a postnatal developmental mismatch between the rates of growth of neural tissue and the osseous posterior fossa rather than a congenital finding.19 Further studies are needed to explore these potential theories of why syrinxes are possibly more prevalent in patients with CSF leaks from vascular malformations, but regardless, this prevalence may be a reason to search for a vascular malformation as a potential etiology for the SIH.
Dural enhancement was absent in all of our patients, which is typically the most prevalent brain MRI feature in SIH.20 We speculate that this imaging sign was absent in our patient cohort due to delayed diagnosis, because it has been reported that dural enhancement can resolve with time, even without treatment.21
Classifying vascular anomalies can be challenging due to historical terms. For example, vascular malformations are often misclassified as hemangiomas, which are vascular tumors. In 1 study that evaluated published articles with the term “hemangioma” in 2009, >70% of hemangiomas were misclassified.22 In our case series, 2 patients had their venous malformation misclassified as a hemangioma. The ISSVA classification of vascular anomalies is subdivided into vascular tumors and malformations. Under the vascular malformation category, there are various types, but venous and lymphatic malformations are the most common. This nomenclature challenge has also been observed in patients with spinal and skull base leaks associated with vascular malformations, making it difficult to surmise the exact incidence because correct terminology is not always used. Moreover, in patients with vascular anomaly syndromes, this nomenclature can be more challenging, given the wide variety and the greater diagnostic complexity. We suggest that patients with CSF leaks from an underlying vascular malformation be referred to a vascular anomalies clinic for care of the vascular malformation, in addition to the CSF leak treatment, if possible, because certain vascular malformations may be amenable to medical therapies, such as mammalian target of rapamycin (mTOR) inhibitors.
In 5 of the 6 patients, there was a direct fistula between the CSF and the vascular malformation, while 1 patient had a CSF-venous fistula that is typical for other patients with SIH without vascular malformations. Prior descriptions of these fistulas have also been labeled CSF-venous fistulas. While this labeling is technically true, because there is a connection between the CSF and the venous system in patients with a venous malformation, we prefer the more technically correct term of CSF-venous malformation fistula. In addition, if there is a lymphatic connection, then CSF-lymphatic malformation fistula is the suggested terminology. This more accurately characterizes the physiology and leads to better tracking for research purposes.
Two of the 6 patients had skull base leaks, specifically in the petrous apex and temporal bone. Skull base leaks do not typically result in SIH, because the spinal level in which the CSF pressure changes from negative to positive relative to the atmospheric pressure is typically in the upper cervical spine. CSF leaks occurring above this zero-pressure and hydrostatic indifference point, therefore, do not typically result in a CSF leak when the patient is upright.23 In 1 study of 273 patients with SIH by Schievink et al,24 there were no cases of skull base leaks. Subsequently, however, this same group reported a single case of a large posterior fossa CSF leak resulting in SIH.25 In a more recent study, 31 patients with skull base leaks had a mean Bern score of 0.9, and the single patient who had dural enhancement had an infratentorial CSF leak.26 We postulate that if a skull base leak is severe, it could possibly overcome the pressure gradient between the brain and spine and result in SIH. Further research is needed to validate this theory.
There are various treatment options for CSF-vascular malformation fistulas. In 3 cases, the fistula was targeted with blood or fibrin glue patches with some relief. In our 1 patient with a more typical CSF-venous fistula, fibrin glue patching was curative. In patient 5 with a CSF-lymphatic malformation fistula, surgery was successfully performed. Surgical treatment is not without risks, however, because 1 reported case of surgery for a spinal CSF-venous malformation fistula was complicated by extensive blood loss.2 In certain cases, transvenous Onyx (Medtronic) embolization may be an option if there is a venous pathway to the fistula. Further studies are needed to determine the treatment efficacy for CSF-vascular malformation fistulas.
CONCLUSIONS
CSF-vascular malformation fistulas are a rare cause of SIH. It had been proposed previously that a CSF leak should be suspected in patients with epidural venous malformations—either syndromic or isolated—if they present with clinical symptoms of SIH.3 We agree with this recommendation but further suggest that any person presenting with headache or other SIH symptoms in whom an underlying vascular malformation syndrome or an isolated paraspinal or skull base vascular malformation is present, should undergo a CT myelogram and/or cisternogram to identify a possible CSF leak into that entity.
Patient demographics of CSF-vascular malformation fistulas
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received July 17, 2024.
- Accepted after revision August 14, 2024.
- © 2025 by American Journal of Neuroradiology