Graphical Abstract
Abstract
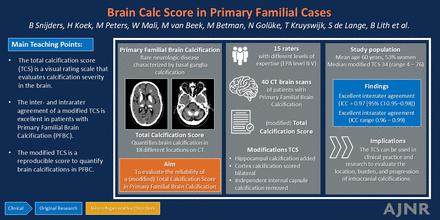
BACKGROUND AND PURPOSE: The total calcification score (TCS) is a visual rating scale to measure primary familial brain calcification (PFBC)–related calcification severity on CT. We investigated the inter- and intrarater agreement of a modified TCS.
MATERIALS AND METHODS: Patients aged ≥18 years with PFBC or Fahr syndrome who visited the outpatient clinic of a Dutch academic hospital were included. The TCS was modified, for example, by adding hippocampal calcification, and ranged from 0 to 95 points. Fifteen raters evaluated all CTs, of whom 3 evaluated the CTs twice. Their entrustable professional activity (EPA) level ranged from II (medical student) to V (neuroradiologist). Agreement was assessed by using the intraclass correlation coefficient (ICC) for the total score. Kendall’s W and weighted Cohen κ were used to determine the inter- and intrarater agreement for individual locations, respectively.
RESULTS: Forty patients were included (mean age 60 years, 53% women). The median modified TCS was 34 (range 4–76). For all EPA levels, the interrater agreement of the modified TCS was excellent (ICC = 0.97 [95% CI 0.95–0.98]). The Kendall W values were good to excellent for commonly affected locations, but poor to moderate for less commonly affected locations for raters with lower levels of expertise. The intrarater agreement of the modified TCS was excellent. The Cohen κ of most locations were substantial to almost perfect.
CONCLUSIONS: The modified TCS can be used with excellent reproducibility of the overall amount of brain calcifications and with limited training, although for some individual calcification locations more expertise is needed.
ABBREVIATIONS:
- EPA
- entrustable professional activity
- IBGC
- idiopathic basal ganglia calcification
- ICC
- intraclass correlation coefficient
- PFBC
- primary familial brain calcification
- TCS
- total calcification score
- UMCU
- University Medical Center Utrecht
SUMMARY
PREVIOUS LITERATURE:
The total calcification score (TCS) is a visual rating scale on CT scans that evaluates calcification severity in different locations of the brain. The TCS has been used in several studies in patients with primary familial brain calcification (PFBC) and Fahr syndrome, which are rare neurologic disorders characterized by basal ganglia calcification. The reproducibility of the TCS has not been evaluated in detail yet. This study evaluated the inter- and intrarater agreement of a modified TCS in a group of raters with different backgrounds and levels of expertise.
KEY FINDINGS:
The modified TCS is an excellent reproducible score to quantify the overall amount of brain calcifications in patients with PFBC and Fahr syndrome, while limited expertise of the rater is needed. For some individual locations more expertise is needed.
KNOWLEDGE ADVANCEMENT:
The score can be used in clinical practice and research to evaluate the location, burden, and progression of intracranial calcifications in patients with PFBC and Fahr syndrome. This study is a next step in the improvement of the diagnostic process for this rare phenomenon.
Primary familial brain calcification (PFBC), also known as Fahr disease or idiopathic basal ganglia calcification (IBGC), is a rare neurologic disorder that is characterized by bilateral basal ganglia calcifications.1 Calcifications often develop in other locations of the brain as well, for example in the thalami, cerebral subcortical white matter, or cerebellum.2 These calcifications may cause symptoms such as cognitive decline, movement disabilities, or neuropsychiatric disorders. The symptoms correlate with the calcification burden in patients with PFBC.2⇓–4
Accurate assessment of calcification location and burden is an important step in the diagnostic process of PFBC.1 Assessment of the calcifications may also play a role in disease monitoring and evaluation of interventions. Objective methods to quantify these calcifications are limited. One method is the total calcification score (TCS), which is a visual rating scale on CT scan that evaluates calcification severity in different locations of the brain.5 The TCS was developed by a French IBGC Study group in 2013. Since then, several studies have used the TCS to quantify the amount of calcifications in patients with PFBC.2⇓–4
Before implementing the TCS in clinical practice or future studies, it is essential to evaluate its reproducibility. The French IBGC Study5 group showed excellent interrater agreement between 2 members of the study group, but further data on inter- and intrarater agreement are lacking. The feasibility of using the TCS by raters with different levels of expertise is also unknown.
After working with the TCS in our patients with PFBC at our specialized clinic, we gained several insights that led to revision of the original TCS. This study aimed to investigate the inter- and intrarater agreement of a modified TCS in patients with PFBC by a group of raters with different levels of expertise.
MATERIALS AND METHODS
Patient Selection
This study was conducted in accordance to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines. Ethical approval was obtained from the local Dutch Medical Ethical Review Committee NedMec (registration numbers 21–170 and 22–1005). All patients gave written informed consent.
In this retrospective study, all patients aged ≥18 years who were diagnosed with PFBC or Fahr syndrome and visited the outpatient clinic of the University Medical Center Utrecht (UMCU), the Netherlands, between September 1, 2019 and July 1, 2023, were eligible for inclusion. The UMCU is an academic hospital with expertise in PFBC and Fahr syndrome. PFBC was diagnosed based on: 1) presence of bilateral basal ganglia calcifications as seen on CT, 2) TCS above the age-specific threshold (original TCS >0 in age <40 years, >4 in age 40 to 60 years, and >5 in >60 years),5 and 3) clinical symptoms consistent with the diagnosis. A genetic mutation associated with PFBC confirmed the diagnosis, but was not a mandatory criterion for inclusion. Patients were diagnosed with Fahr syndrome if they met the aforementioned criteria for PFBC, but a secondary cause associated with bilateral basal ganglia calcification was identified, for example, endocrine disorders such as hypoparathyroidism.1 All consecutive patients who were scanned in an outpatient setting according to the PFBC CT scanning protocol in the UMCU were included in this study. Patients were excluded if they were scanned according to a different CT scanning protocol or in another hospital.
CT Imaging
The CT scanning protocol was an unenhanced scan of the full brain at 120 voltage (peak) (kVp) and 250 milliampere. All scans were acquired at dual-layer detector scanners (Iqon, CT7500, Philips Healthcare). The scan data were reconstructed at a slice thickness of 0.9 mm by using iterative model-based reconstruction. When a patient was scanned multiple times during the study period, the most recent scan was included.
Modified TCS
The original TCS is a visual rating scale that quantifies the amount of calcification in 18 different locations in the brain: left and right lenticular nucleus, left and right caudate nucleus, left and right thalamus, left and right cerebral subcortical white matter, cerebral cortex, left and right cerebellar hemisphere, vermis, left and right midbrain, pons, medulla, and left and right internal capsule only if independent of other calcifications. Each location gets attributed a score ranging from 0 to 5: 0 = no calcification; 1 = punctate; 2 = faint; 3 = moderate; 4 = severe; and 5 = severe and confluent. Examples of CT scans with different degrees of calcification in several locations are included in the article that developed the TCS.5 The TCS is the sum of score points of all locations and this overall score ranges from 0 to 90 points.5
After working with the original TCS in previous research, we updated the original TCS by modifying 3 aspects.2 First, we removed the items “independent internal capsule calcification left and right” from the score. Independent internal capsule calcifications have not been observed in patients with PFBC or Fahr syndrome before on CT, neither in our study population (assessed by raters B.M.G.S. and P.A.d.J.), nor in the literature.2,5 When the internal capsule is calcified, these calcifications are always confluent with calcifications in nearby locations and never independent.2,5 Second, we divided the item “cerebral cortex calcification” into a left and right cerebral cortex, which is in accordance with the other locations that are already scored separately. Third, we added the items “hippocampal calcification left and right” to the score. We commonly observed hippocampal calcification in PFBC in our clinical practice, but the hippocampus is not included in the original TCS. Hippocampal calcifications were scored bilaterally as: 0 = no calcification; 1 = punctate; 3 = moderate; or 5 = severe. We did not include score 2 and 4 because the hippocampus is too small to quantify the calcifications on a 5-point scale. Examples of the hippocampal calcification score are shown in the Online Supplemental Data. An example of the modified TCS scoring form is shown in the Online Supplemental Data. The modified TCS ranges from 0 to 95 points.
CT Scan Assessment
Fifteen raters with different backgrounds and levels of expertise from the UMCU visually assessed 40 consecutive CT scans of the brain of 40 individual patients on 120 kVp reconstructions at least once, by using the modified TCS. Level of expertise for evaluating neurologic CT scans was predetermined based on the entrustable professional activity (EPA) level: I = has knowledge; II = may act under full supervision; III = may act under moderate supervision; IV = may act independently; and V = may act as supervisor and instructor.6 EPA level V applied to residents or radiologists with extensive experience in neuroradiology. Among the raters were: 3 radiologists (D.R.R., P.A.d.J., W.P.T.M.M., EPA levels V, IV, and IV, respectively), 3 radiology residents (M.M.v.B., S.V.d.L., S.M.U.V., EPA level IV, V, and III), 1 geriatrician (N.M.S.G., EPA level II), 1 internist (M.J.L.P., EPA level II), 5 geriatric residents (B.M.G.S., M.M.v.B., M.J.R., R.M.P., T.K., all EPA level II), 1 internal medicine resident (W.R.V., EPA level II), and 1 final-year medical student (B.D.W.T.L., EPA level II). Each rater received an instruction manual including an overview of the anatomy of the brain and examples of calcification scores in different brain regions, the latter was adapted from the developmental study of the TCS.5 Examples of hippocampal calcification were collected from a previous study that evaluated hippocampal calcification in a cohort of memory clinic patients.7 Three raters (B.M.G.S., P.A.d.J., W.P.T.M.M.) scored the 40 scans twice with a minimum time interval of 3 months between the first and second assessment to determine the intrarater reproducibility. During the second assessment, raters were blinded for scores of the first assessment. The scores of the first assessment were used to determine the interrater agreement. Each scan was examined in axial, coronal, and sagittal view. All raters were blinded for clinical variables.
Statistical Analysis
Data were collected based on the date of scanning and included: age, sex, diagnosis, and results of genetic testing (if available). Data were presented by using number with percentage for categoric variables, mean with standard deviation for continuous nonskewed variables, and median with interquartile range for skewed variables. Prevalence of calcified brain locations was calculated based on the scores of the rater with the highest level of expertise and most years of experience in neuroradiology (neuroradiologist D.R.R.). The interrater agreement for the overall score of the modified TCS and for the subscore of each individual item was assessed by using the intraclass correlation coefficient (ICC) with its 95% CI and Kendall concordance coefficient W, respectively. The interrater agreement was determined for all raters and per level of expertise. The intrarater agreement was assessed by using the ICC and weighted Cohen κ, respectively, with their 95% CIs.8 An ICC of <0.5 can be interpreted as poor, of 0.5–0.75 as moderate, of 0.75–0.9 as good, and of >0.90 as excellent agreement.9 Similarly, a weighted Cohen κ of <0.0 can be interpreted as poor, of 0.0–0.2 as slight, of 0.21–0.4 as fair, of 0.41–0.6 as moderate, of 0.61–0.8 as substantial, and of 0.81–1.0 as almost perfect.10 There is no general agreement on cutoff values to interpret Kendall W, which ranges from 0 (no agreement) to 1 (complete agreement). The higher the W score, the better the overall agreement.8 For the purpose of this study, the W scores were interpreted in the same way as the ICC (which also ranges from 0 to 1 and is interpreted to conform to the same rule “the higher, the better”). Statistical analyses were performed by using SPSS Statistics for Windows, version 29.0 (IBM).
RESULTS
Forty patients were included in this study. Baseline characteristics are presented in Table 1. The mean age was 59.9 years (standard deviation 14.7) and 52.5% were women. Most patients (95%) were diagnosed with PFBC. A genetic mutation was found in one-half of the patients who underwent genetic testing. The SLC20A2 gene was the most commonly affected gene.
Baseline characteristics of patients with primary familial brain calcification or Fahr syndrome
According to the ratings of neuroradiologist D.R.R. (EPA level V), the modified TCS ranged from 4 to 76 points (median 34, IQR 15–54). The most common affected locations included: both lenticular nuclei (affected in 100% of patients), 1 or both cerebellar hemispheres (affected in 70%), 1 or both thalami (affected in 68%), left and right subcortical white matter (affected in 65%), and 1 or both caudate nuclei (affected in 63%). Hippocampal calcification was present in 24 patients (60%), of whom 19 (79%) had bilateral and 5 (21%) unilateral calcification. In the 80 hippocampi of 40 included patients, calcifications were absent in 37 (0 points, 46%), punctate in 15 (1 point, 19%), moderate in 11 (3 points, 14%), and severe in 17 (5 points, 21%) hippocampi. In patients aged <60 years, 6 out of 17 patients (36%) had calcifications in 1 or both hippocampi, compared with 18 out of 23 patients (78%) aged ≥60 years. Cerebral cortex calcifications were observed in 18 patients (45%), of whom 15 (83%) had bilateral and 3 (17%) had unilateral calcifications. Calcifications in the left and/or right mesencephalon, pons, and medulla were scarce (present in 10%, 10%, and 3% of patients, respectively). Independent internal capsule calcifications were not observed. The prevalence of calcification per location is shown in the Online Supplemental Data.
Interrater Agreement
The interrater agreement for the modified TCS and each individual location is shown in Table 2. The ICC of the overall score of the modified TCS was excellent for all raters (0.96; 95% CI 0.94–0.98), for raters within the same EPA level, and for all comparisons between 2 individual raters (Table 2 and Online Supplemental Data). For the most commonly affected locations (lenticular nucleus, caudate nucleus, thalamus, cerebral subcortical white matter, cerebral cortex, and cerebellar hemisphere), the interrater agreement was good to excellent, although Kendall W increased with the level of expertise in most locations. The interrater agreement in raters with lower levels of expertise was poor to moderate in areas that were less frequently affected (hippocampus, vermis, mesencephalon, medulla), while good to excellent in raters with a high level of expertise.
Interrater agreement of the modified total calcification score, for all raters and by level of expertise
Intrarater Agreement
The intrarater agreement of the overall score of the modified TCS was excellent for all 3 raters (ICC ranging from 0.96 to 0.99). The Cohen κ of almost all locations were substantial to almost perfect. The intrarater agreement was only moderate for the hippocampus for rater W.P.T.M.M., poor for the mesencephalon for rater B.M.G.S., and poor for the medulla for rater W.P.T.M.M. Table 3 presents the intrarater agreement for the modified TCS and each individual location.
Intrarater agreement of the modified total calcification score
DISCUSSION
In this study, we updated the TCS by removing the items “independent internal capsule calcification left and right,” by dividing the item “cerebral cortex calcification” into left and right, and by adding the items “hippocampal calcification left and right.” We assessed the inter- and intrarater agreement of the modified score in patients with PFBC and Fahr syndrome. The interrater agreement of the overall score was excellent and it could be accurately established by raters with both lower and higher levels of expertise. There was a high level of agreement between raters regarding frequently affected brain locations like the lenticular nucleus or thalamus. Variability between raters with limited expertise increased for locations that were less often affected. The intrarater agreement was excellent for the overall score and substantial to almost perfect for most brain regions, indicating a good reproducibility.
Our findings are reassuring for the use of the TCS and modified TCS, which is in line with results of the French IBGC Study group5 that brain calcifications can be scored with high interrater agreement. They reported a weighted Cohen κ of 0.97 for the original TCS.5 In addition to the previous study, we evaluated the agreement for all locations separately and tested raters with various expertise levels. Our findings are a valuable addition to the limited knowledge of the measurement of intracranial calcifications in PFBC.
To our knowledge, this is the first time the prevalence of hippocampal calcification has been described in patients with PFBC and Fahr syndrome. The prevalence of calcification in the other intracranial locations in PFBC has been described before.2,4 Hippocampal calcification can also be seen as incidental finding on CT scans in the general population. For example, in an Australian hospital study that randomly included 300 patients who underwent nonenhanced brain CT scans, hippocampal calcifications were present in 22% of patients aged over 50 years. No hippocampal calcifications were observed in patients <50 years of age.11 Another study, which examined brain CT scans of 1130 patients with (suspected) acute ischemic stroke, reported a prevalence of hippocampal calcification of 8% in patients <40 years. This prevalence gradually increased with age up to 45% in patients >80 years.12 In a cohort including 1991 patients visiting a memory clinic (mean age 78 years), 19% had calcifications in the hippocampus on brain CT scan.13 The prevalence in our study population (36% in <60 years, 78% in ≥60 years) is considerably higher compared with these studies, suggesting that patients with PFBC or Fahr syndrome have a higher risk of developing hippocampal calcification. However, we do not know whether this higher prevalence of hippocampal calcification in patients with PFBC and Fahr syndrome is primarily due to the pathophysiology of the disease or due to the presence of other risk factors. Given this higher prevalence of hippocampal calcification, we decided to add the hippocampus to the modified TCS to increase awareness for this frequently affected brain location.
A strength of our study is that we included a large group of raters with different backgrounds and levels of expertise. We evaluated the reproducibility and feasibility of the score in a relatively large group of patients with intracranial calcifications. We provided the raters with written instructions only, which appeared to be sufficient to adequately assess the calcification scores. Furthermore, we enhanced an existing brain calcification score by adding several relevant items (bilateral cortex and hippocampus calcification) and removing a less relevant item (independent internal capsule calcification). The modified TCS obtains a more complete overview of all calcified brain locations compared with the original score. This is a next step in the improvement of the diagnostic process for this rare disease. However, the clinical relevance of calcification per specific brain location is still largely unknown. A recent study has demonstrated that calcifications in the lentiform nucleus and subcortical white matter are associated with motor and cognitive decline, respectively.14 More research is needed in larger populations to evaluate the clinical relevance of other calcified brain locations.
Our study must be interpreted in the light of some limitations. First, we had a small number of raters with higher levels of expertise, yet this might have led to an underestimation of the reproducibility. One of our raters with EPA level II (N.M.S.G.) had previous experience with administering the original TCS.2 Yet, none of the other readers had previous experience with the TCS. Therefore, reproducibility of calcification scoring with the (modified) TCS at another institution is likely achievable with the methodology of training and scoring as applied in the current study. Next, we modified the TCS without validating the cutoff values for pathologic calcification.4 The French IBGC Study Group, which developed the original TCS, proposed age-dependent cutoff values for the original TCS to distinguish patients with normal amounts of intracranial calcification due to the natural ageing process from patients with pathologic calcification.5 These cutoff values need to be validated for our modified score. Because hippocampal calcification is prevalent in the general population, especially among older adults, cutoff values for the modified TCS will likely need to be raised. Therefore, caution is needed with applying the existing cutoff values to the modified TCS. These values might not be able to adequately differentiate between physiologic and pathologic brain calcification when the modified score is used. However, our modified TCS can be used to assess the calcification location, burden and progression in a more detailed and complete way in known patients with PFBC and with Fahr syndrome. Further research is needed to reestablish cutoff values of the modified TCS, before it can be used to differentiate between physiologic and pathologic calcification load. Last, we used a single scanning protocol for this study. Reproducibility of the score may suffer when thicker slices are obtained or when contrast-enhanced scans are used.
CONCLUSIONS
The modified TCS is an excellent reproducible score to quantify the overall amount of brain calcifications in patients with PFBC and Fahr syndrome, while limited expertise of the rater is needed. For some individual locations more expertise is needed. The score can be used in clinical practice and research to evaluate the location, burden, and progression of intracranial calcifications in patients with PFBC and Fahr syndrome.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received May 28, 2024.
- Accepted after revision July 19, 2024.
- © 2025 by American Journal of Neuroradiology