Abstract
BACKGROUND AND PURPOSE: Alterations of the basilar artery (BA) anatomy have been suggested as a possible MRA feature of Fabry disease (FD). Nonetheless, no information about their clinical or pathophysiologic correlates is available, limiting our comprehension of the real impact of vessel remodeling in FD.
MATERIALS AND METHODS: Brain MRIs of 53 subjects with FD (mean age, 40.7 [SD, 12.4] years; male/female ratio = 23:30) were collected in this single-center study. Mean BA diameter and its tortuosity index were calculated on MRA. Possible correlations between these metrics and clinical, laboratory, and advanced imaging variables of the posterior circulation were tested. In a subgroup of 20 subjects, a 2-year clinical and imaging follow-up was available, and possible longitudinal changes of these metrics and their ability to predict clinical scores were also probed.
RESULTS: No significant association was found between MRA metrics and any clinical, laboratory, or advanced imaging variable (P values ranging from −0.006 to 0.32). At the follow-up examination, no changes were observed with time for the mean BA diameter (P = .84) and the tortuosity index (P = .70). Finally, baseline MRA variables failed to predict the clinical status of patients with FD at follow-up (P = .42 and 0.66, respectively).
CONCLUSIONS: Alterations of the BA in FD lack of any meaningful association with clinical, laboratory, or advanced imaging findings collected in this study. Furthermore, this lack of correlation seems constant across time, suggesting stability over time. Taken together, these results suggest that the role of BA dolichoectasia in FD should be reconsidered.
ABBREVIATIONS:
- BA
- basilar artery
- FA
- fractional anisotropy
- FASTEX
- FAbry STabilization indEX
- FD
- Fabry disease
- Gb3
- globotriaosylceramide
- ICC
- intraclass correlation coefficient
- LysoGb3
- globotriaosylsphingosine
- MSSI
- Mainz Severity Score Index
- PCA
- posterior cerebral artery
- TI
- tortuosity index
SUMMARY
PREVIOUS LITERATURE:
The search for a reliable MR biomarker in FD is an unmet need. Different neuroradiologic features have been reported in these patients, but none of them has proved to be, to date, reliably useful for either diagnostic or prognostic purposes. In this context, alterations of the posterior circulation (namely elongation, tortuosity, and ectasia, especially of the BA) have been proposed as the most prominent neuroradiologic findings in FD, but the real prevalence of this finding as well as the possible clinical consequences or pathophysiologic correlates are still debated in literature.
KEY FINDINGS:
No meaningful increase in the mean BA diameter was observed in our sample. No significant correlation emerged between morphologic alterations of the BA and the macro- or microstructure of the posterior circulation as well as any clinical outcome, either at a cross-sectional or longitudinal evaluation.
KNOWLEDGE ADVANCEMENT:
BA measurement is prone to a high variability both within and between readers, mitigating the routine use in clinical practice of this feature. Furthermore, the absence of correlation with clinical markers of the disease excludes a possible use of the BA abnormalities as a prognostic biomarker in FD.
Fabry disease (FD) is a rare X-linked lysosomal storage disorder caused by deficient activity of the lysosomal enzyme α-galactosidase A,1 which leads to a progressive lysosomal accumulation of glycosphingolipids, mainly globotriaosylceramide (Gb3) and its deacylated form globotriaosylsphingosine (LysoGb3), in multiple organs including the CNS.2
Although a relatively new-but-large amount of evidence suggests the occurrence of a deep and widespread CNS involvement occurring in FD,3⇓⇓⇓⇓-8 brain damage has historically been considered as primarily sustained by cerebral vasculopathy, due to the high incidence of stroke in these patients.9,10 In this light and from a neuroradiologic standpoint, much interest in the field has been, therefore, dedicated to the search for an imaging biomarker related to vascular abnormalities in FD.11 Among these, the dilative arteriopathy of the posterior circulation is recognized as one of the main conventional imaging findings in this condition, with reported alterations of the vertebrobasilar system that included elongation, ectasia, tortuosity, and focal aneurysmal dilation.11,12 In the search for quantitative biomarkers to characterize and standardize the evaluation of these posterior circulation alterations, different measurements of the dolichoectasia of the basilar artery (BA) have been tested, in some cases proposing thresholds to differentiate patients with FD from healthy controls.13,14 However, this evidence is described in small groups of patients13,15 and not always confirmed in other studies, with conflicting results reported in the literature.11,14,16⇓-18 Furthermore, when tested in large and representative groups of patients,14,18⇓-20 neither information about possible association with structural alterations of the posterior circulation territories nor the possible clinical impact of this alteration was ever probed, leaving the question of the real impact of this observed change in FD unanswered.
Given this background, this study had 2 main aims: 1) to replicate the methodology used in BA measurements previously proposed in the FD literature, and 2) to evaluate the suitability of these measurements as severity biomarkers in this condition. To fulfill this second major aim, we a) investigated the relation between BA changes and the macro- and microstructure of the posterior circulation territories, as well as the possible associations with clinical and laboratory metrics to understand both the pathophysiologic and biologic meanings of posterior circulation alterations in FD and b) investigated these variables in a longitudinal setting, to evaluate the occurrence of possible changes over time and their meaning in FD and if they can predict the clinical status of the patients.
MATERIAL AND METHODS
Participants
In this retrospective study, part of a larger monocentric framework of CNS involvement in FD, MRI, and clinical data were collected from October 2015 to December 2019. From a clinical standpoint, exclusion criteria were the following: age younger than 18 years and older than 65 years and a history of major cerebrovascular events and/or other relevant neurologic or systemic conditions. Furthermore, patients with artifacts on MRI sequences evaluated in this analysis, an incomplete MRI examination, or vertebral artery agenesia on MRA were excluded from the study (Fig 1).
Flow chart showing how the final number of patients included in this study was reached. VA indicates vertebral artery.
Clinical and laboratory variables collected within 1 week of the MRI scan and retrieved from the medical records included the Mainz Severity Score Index (MSSI)21 and the FAbry STabilization indEX (FASTEX)22 scores as indices of multiorgan damage severity, along with the residual α-galactosidase A activity and LysoGb3 levels. According to the median value of the MSSI score found in our group, patients were defined as mildly (if equal or minor to the median value) or severely affected. A similar approach was applied for the neurologic subscore of the MSSI, to identify patients with a less severe or a more pronounced CNS involvement.
This study was approved by the local Ethics Committee (no 62/10), “Carlo Romano”, University of Naples “Federico II” in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments. Written informed consent was obtained from all subjects before enrollment.
MRI Data Acquisition
All participants underwent a standardized MRI protocol on the same 3T scanner (Magnetom Trio; Siemens), with the same software version (VB19) and the same 8-channel head coil. The MRI protocol included an MPRAGE: 160 axial slices, TR = 1900 ms, TE = 3.4 ms, TI = 900 ms, flip angle = 9°, voxel size = 1.0 × 1.0 × 1.0 mm3 for the macrostructural evaluation of brain volumes; a 3D FLAIR: 160 sagittal slices, TR = 6000 ms, TE = 396 ms, TI = 2200 ms, voxel size = 1.0 × 1.0 × 1.0 mm3 used for the assessment of white matter hyperintensities; a diffusion-weighted spin-echo sequence: TR = 7400 ms, TE = 88 ms, flip angle = 90°, voxel size = 2.2 × 2.2 × 2.2 mm3 with 64 directions at b = 1000 s/mm2 in addition to nine b = 0 s/mm2 for the evaluation of brain microstructure; and a 3D TOF-MRA sequence: 128 slices, TR = 22 ms, TE = 3.86 ms, flip angle = 18°, voxel size = 1.1 × 0.8 × 0.8 mm3 used for the posterior circulation anatomy assessment.
MRI Data Analysis
All MRA images were evaluated by 3 readers with different expertise: a neuroradiologist resident with 2 years of experience in neuroimaging (reader A) and 2 board-certified neuroradiologists with >5 (reader B) and 15 years of experience (reader C), respectively. Readers evaluated all TOF images independently and blinded to the subjects’ demographic and clinical data, to evaluate the reliability of MRA-related signs in FD and a possible effect of the degree of experience in these evaluations. Measures obtained by the most experienced rater (reader C) are reported in the following sections of the article and used for all statistical analyses. Furthermore, a subgroup of 15 subjects was randomly selected to assess the intrareader reliability of the investigated MRI measures after a wash-out period of 15 days by reader A.
According to a previous study,14 2 measures were obtained from the MRA data: the mean BA diameter and its tortuosity index (TI). These 2 metrics were calculated as follows: using the MPR function of a freely available DICOM viewer (Horos, Version 3.3.6; http://horosproject.org), TOF reformats were oriented perpendicular to the BA at 3 different points (namely, proximal, central, and distal portions) to obtain the corresponding diameters. An average value of these 3 measurements was then calculated, with the mean BA diameter used in all analyses. Using the same MPR function, we selected the coronal plane, with a MIP reconstruction and the minimum slab thickness enabling the inclusion of the BA in its entirety. On this image, the linear and curved lengths of the BA were collected as a straight line passing from the apex of the BA to the convergence of the vertebral arteries and as a curved line following the vessel along its central portion in its entirety, respectively. These 2 measurements were then combined to calculate the TI according to the formula [TI = (curved length/linear length) – 1]. An example of these measurements is available in Fig 2.
An example of the posterior circulation vessel measurements evaluated in this study. In the left panel (A), an MIP coronal MPR of the TOF sequence acquired in this study shows how the curved (thick dashed black line) and linear length (straight black line) measures were traced with these 2 measures that were combined to calculate the TI. In the right panels, 3 axial MPRs at 3 different levels of the BA (highest, B; intermediate, C; and lower, D, portions corresponding to the thin dashed black lines in A), show where the 3 axial diameters of this vessel were obtained to calculate the mean BA diameter for each patient.
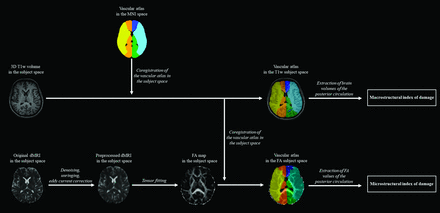
Macro- and microstructural indices of damage in FD were obtained as follows: As a first step, DICOM images were converted into a NIfTI format using dcm2niix (https://github.com/rordenlab/dcm2niix) to be processed by using FSL (https://fsl.fmrib.ox.ac.uk/fsl). For the macrostructural evaluation, brain volumes were segmented using the FMRIB Automated Segmentation Tool (FAST; https://github.com/scitran-apps/fsl-fast)23 into their 3 major components: gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF). For all subsequent analyses, GM and WM volumes were considered together, normalized for the total intracranial volume (as the sum of GM, WM, and CSF), to take the head size into account. For the microstructural evaluation, diffusion MR images underwent denoising, unringing, and eddy current correction, followed by tensor fitting to compute fractional anisotropy (FA) maps as the main index of microstructural damage.24⇓⇓-27 The obtained FA maps were linearly registered to 3D T1-weighted volumes, with these latter registered to the standard Montreal Neurological Institute (MNI) space through a 2-stage registration process, with a linear followed by a nonlinear transformation. The corresponding transformation matrices were then inverted to register an atlas of the vascular territories available in the MNI space to subject-level scans28,29 and to extract brain volumes and mean FA values of the posterior cerebral artery (PCA) territory.28,29 A graphic representation of these processing steps is available in Fig 3.
Workflow of the main processing steps performed to obtain macro- and microstructural indices of damage of the posterior circulation. T1w indicates T1-weighted; dMRI, diffusion MR imaging.
Statistical Analysis
To assess both the inter- and the intrareader agreements, we performed a 2-way mixed intraclass correlation coefficient (ICC) analysis with absolute agreement. According to the literature,30 values >0.9 were interpreted as a proxy for an excellent reliability, while values <0.5 were indicative of poor reliability. Values ranging from 0.5 to 0.75 and from 0.75 to 0.9 were interpreted as indices of moderate and good reliability, respectively.
Possible correlations between MRA metrics and demographic (age), clinical (MSSI at baseline, FASTEX at follow-up), laboratory (residual α-galactosidase A activity and LysoGb3 levels), and imaging (global Fazekas score as well as volumes and FA of the posterior circulation) variables were probed via partial correlation analysis, age- and sex-corrected as appropriate.
Possible differences in terms of MRA variables between patients with FD with a less pronounced or more severe clinical involvement, either according to the general MSSI scale or its neurologic subscore, were probed via general linear model, age- and sex-corrected.
In the subgroup of subjects with a follow-up MR scan available, possible differences in terms of BA diameters and TI between baseline and follow-up data were probed via paired t tests. Furthermore, we tested whether MRA variables at baseline were independent predictors of the FASTEX score recorded at the follow-up examination, to investigate the possible prognostic role of these measures, via linear regression analysis. Finally, the possible association between MRA metrics and macro- and microstructural indices of damage of the posterior circulation were tested, similar to the baseline data, via partial correlation analysis, age- and sex-corrected.
All statistical analyses were performed using the SPSS (Statistical Package for Social Sciences, Version 25.0; IBM) software and corrected for multiple comparisons via Bonferroni correction with a statistical threshold equal to 0.0045 (as 0.05/11, given that we probed 11 different variables: age, mean BA diameter, TI, MSSI scale and its neurologic sub-score, FASTEX score, residual enzyme activity, LysoGb3 levels, Fazekas score, and macro- and microstructural metrics of the PCA vascular territories).
The Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) checklist (https://cdn.amegroups.cn/journals/pbpc/files/journals/3/articles/67393/public/67393-PB4-4751-R1.pdf) for this study can be found in the Online Supplementary Data.
RESULTS
A final number of 53 subjects with FD (age, 40.7 [SD, 12.4] years, male/female ratio = 23:30), all with classic or late-onset pathogenic mutations according to ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) and all under enzyme-replacement therapy (44/53, 83.0%) or chaperone therapy (5/53, 9.4%) with the exception of only 4 patients (4/53, 7.6%) were included in this study (Fig 1). For a subgroup of 20 patients (42.1 [SD, 10.1] years at baseline, male/female ratio = 9:11), a follow-up MR scan was also available (mean follow-up time, 28 [SD, 8] months). Demographic and clinical data of the subjects included in the analyses are available in Table 1.
Demographic and clinical data of the subjectsa
The ICC analysis of the BA diameter showed a moderate agreement between the less-experienced reader and the remaining 2 (0.69 and 0.72, respectively), with this value increased to 0.87 (suggesting a good-but-not-excellent agreement) between the 2 more experienced readers. On the other hand, the ICC analysis of the TI had a poor reliability of this measure between the less-experienced reader and the remaining 2 (0.34 and 0.46, respectively). Similar to the findings of the BA diameter analysis, this value increased to a moderate agreement (0.65) between the 2 more-experienced readers. Finally, the intrareader ICC analysis showed a moderate agreement (0.65) for the mean BA diameter, with an excellent agreement (0.99) for the TI. Results of this analysis can be found in the Online Supplemental Data.
In the whole group of patients with FD, we found a mean BA diameter equal to 3.3 [SD, 0.5] mm, with this metric showing no significant correlation with age (P = .01, ρ = 0.35). The mean curved and linear lengths of the BA were 29.8 [SD, 5.0] mm and 28.7 [SD, 4.4] mm, respectively, with a resulting mean TI of 0.04 [SD, 0.04] not showing a significant correlation with age (P = .06, ρ = 0.26). Patients with a more severe disease (according to the MSSI) were not different in terms of mean BA diameter (P = .08) or TI (P = .03) compared with subjects with a relative multiorgan preservation. Similarly, patients with FD with a more pronounced CNS involvement were not different compared with subjects with a less severe neurologic phenotype for any of the 2 tested MRA variables (P = .19 and P = .32, respectively).
When the possible pathophysiologic and clinical counterparts of mean BA diameter changes were probed, no significant correlations were found between this value and the global Fazekas score (P = .18, ρ = 0.19), as well as macro- and microstructural metrics of the PCA vascular territories, expressed, respectively, as volumes (P = .51, ρ = 0.09) and mean FA values (P = .85, ρ = −0.03). Similarly, no significant correlations were found between the TI and global Fazekas score (P = .81, ρ = 0.03), mean FA values (P = .09, ρ = 0.24), and volumes (P = .22, ρ = −0.17).
When the possible clinical counterpart of posterior circulation abnormalities was probed, no significant correlations were found between the BA diameter and the MSSI neurologic subscore (P = .18, ρ = 0.19), FASTEX score (P = .06, ρ = 0.27), residual enzyme activity (P = .40, ρ = −0.12), or LysoGb3 levels (P = .03, ρ = 0.31), while a significant correlation was found with the general MSSI scale (P = .003, ρ = −0.40). Similarly, no correlations emerged between the TI and MSSI scale (P = .34, ρ = 0.14), the MSSI neurologic subscore (P = .45, ρ = 0.11), the FASTEX score (P = .19, ρ = 0.19), residual enzyme activity (P = .44, ρ = −0.11), or LysoGb3 levels (P = .68, ρ = −0.06).
In the subgroup of subjects with available follow-up MRI scans, MRA metrics showed a substantial stability across time, without a significant difference between baseline and follow-up examinations for both the mean BA diameter (P = .84) and TI (P = .70). Furthermore, the linear regression analysis showed that neither the BA diameter (P = .42, β = 0.20, [95% CI, −0.47–1.06]) nor the TI (P = .66, β = 0.11, [95% CI, −7.81–11.92]) at baseline proved to be an independent predictor of the FASTEX score at follow-up. Finally, no correlations were found between mean BA diameter values and both macro- and microstructural metrics of the PCA vascular territories, expressed, respectively, as volumes (P = .82, ρ = 0.06) and mean FA values (P = .62, ρ = 0.13). A similar result was found when the TI was correlated with volumes (P = .99, ρ = −0.002) and mean FA values (P = .13, ρ = 0.37) of the posterior circulation.
A complete list of the results of the MRI evaluation with linear measurements collected by the 3 readers is shown in Table 2.
Results of the MRI analysesa
DISCUSSION
This study investigated the presence of morphologic alterations of the vertebrobasilar system in patients with FD, applying several measurements previously proposed in the literature but failing to demonstrate any significant correlation between morphologic alterations of the BA and the macro- or microstructure of the posterior circulation, as well as any clinical outcome, either at a cross-sectional or longitudinal evaluation.
The search for a reliable MR biomarker in FD is an unmet need. Different neuroradiologic features have been reported in these patients, but none has proved to be, to date, reliably useful for either diagnostic or prognostic purposes.11 These include the pulvinar sign, which went from being considered pathognomonic of FD to being today accepted as a rare nonspecific sign of this condition,31,32 and the common, but extremely aspecific, WM hyperintensities, also in the light of some recent literature showing widespread microstructural WM changes extending beyond conventional MRI hyperintensities.3,5,8 In this context of an absence of reliable imaging biomarkers, alterations of the posterior circulation (namely elongation, tortuosity, and ectasia, especially of the BA) have been proposed and relatively accepted as the most prominent neuroradiologic findings in FD. These have been hypothesized to be related to an accumulation of Gb3 within the arterial smooth-muscle cells that may cause a change in the nitric oxide pathway, which, in turn, may lead to a progressive malfunction of the media layer with direct repercussions on vessel anatomy.33,34 The real prevalence of this finding is still debated in the literature, due to the rarity of the disease as well as the lack of standardization about acquisition protocols and/or image-processing. Furthermore, there is still a lack of information about the possible (if any) clinical consequences or pathophysiologic correlates of this alteration. This study fits into this framework with the aim of evaluating, in a large sample, whether morphologic measurements of the BA in FD are reliable among readers with different expertise and then investigating the correlation between changes in BA diameters and clinical data and macro- or microstructural changes in the posterior circulation both cross-sectional and across time.
In contrast with previous literature,14 no meaningful increase in the mean BA diameter was observed in our sample. Several explanations can be found for this result, with the first being the use of a different software for image evaluation (ie, one freely available in our study versus a commercially available software in the study by Manara et al14). Furthermore, in our study, a correction for multiple comparisons was used to reduce the possibility of type I errors. Finally, TOF sequences in this study were acquired at 3T, compared with those acquired at 1.5T, with the known better quality of these images acquired at higher field strengths.35 A reliable biomarker for both diagnostic and prognostic purposes must be easily accessible and reproducible across centers, characteristics that do not seem to emerge for BA evaluation in the replicative section of our study. Indeed, technical validation is one of the crucial steps in the roadmap to follow to identify a possible biomarker.36 Furthermore, reproducibility is a potential issue, given that measurements performed using different equipment, different software, or operators, or at different sites and times should not show any significant variation in order to be considered as a reliable biomarker.36 In this light, our results suggest that the BA measurement is prone to a high variability both within and between readers, further mitigating the routine use in clinical practice of this MR feature.
Besides these considerations on BA diameters and tortuosity advocating against the interpretation of posterior circulation changes as a typical feature of FD, we investigated the possible pathophysiologic meaning of vessel abnormalities and failed to find any possible association between BA measurement and both macro- and microstructural changes of the posterior circulation parenchymal territories. It has been widely demonstrated that in cerebrovascular conditions, MRI is very sensitive in highlighting alterations in both the brain macrostructure (as in stroke37,38) and microstructure (as in cerebral small-vessel disease39,40). Absence of a correlation with these indices of damage, indirectly supported by an MR perfusion study that demonstrated the absence of an increased flow in the posterior circulation areas41 was also found in the longitudinal analysis, in which a lack of significant changes over time in BA metrics was also coupled with a substantial stability of GM and WM structures in the posterior circulation. If the stability across time of the BA diameter and posterior GM volumes is in line with previous evidence,42,43 a recent study showed the occurrence of changes in the ADC maps over time.44 Nonetheless, in this study, maps derived from a more comprehensive technique such as DTI were used, with ADC values that are known to have a relatively lower reliability compared with FA.45 Furthermore and different from previous studies, all acquisitions in our study were performed using the same scanner, with the same software version, coil, and acquisition protocol, thus virtually nullifying any possible bias deriving from these technical issues. Nonetheless, because significant WM microstructural damage is known to occur in this condition,3,5,8,46 future longitudinal studies are warranted to more thoroughly understand the role of these changes over time, given that diffusion MR imaging has proved to be a sensitive biomarker for monitoring the progression of WM damage.47
Finally, the absence of a correlation with clinical and biochemical markers of the disease, either at a cross-sectional and longitudinal evaluation, excludes a possible use of the investigated vessel abnormalities as a prognostic biomarker.
Despite its several strengths, this study presents some limitations, with the first being a lack of a control group, preventing us from defining the exact extent of how much the BA diameter might have differed in patients with FD. Nevertheless, our thorough examination of possible clinimetric counterparts of these posterior circulation changes does not rely on the evaluation of a control group, with the inclusion of a longitudinal analysis that even further mitigates this limitation. Another possible setback of this study might derive from the exclusion of patients with stroke and TIA, potentially leading to a selection bias toward patients with less-pronounced brain involvement. Nonetheless, stroke is associated with an increased BA diameter also in subjects without FD,48 a result that also partly questions whether the previously observed increased BA diameter in FD might be related to a “general” vasculopathy, as the one found in subjects without FD affected by a stroke. Finally, another intrinsic limitation of this work resides in the challenges derived from measuring vessels on MRA without intensity-normalization or procedures for vessel wall identification.14
CONCLUSIONS
Our results suggest that evaluation of the posterior circulation anatomy in FD might not provide a useful or reliable biomarker in this condition. These metrics show a low reliability within the same reader and between readers of different expertise and a lack of macro- and microstructural brain correlates, and they do not show a significant association with clinical findings at either a cross-sectional or a longitudinal evaluation. Therefore, the search for a new, possible quantitative, diagnostic, and prognostic neuroradiologic biomarker in FD remains an urgent need.
Footnotes
A. Scaravilli and S. Capasso contributed equally to this Work.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.
- 30.
- 31.
- 32.
- 33.
- 34.
- 35.
- 36.
- 37.
- 38.
- 39.
- 40.
- 41.
- 42.
- 43.
- 44.
- 45.
- 46.
- 47.
- 48.
- Received June 3, 2024.
- Accepted after revision June 28, 2024.
- © 2024 by American Journal of Neuroradiology