Abstract
BACKGROUND AND PURPOSE: The circle of Willis (COW) is a crucial mechanism for cerebral collateral circulation. This proof-of-concept study aims to develop and assess an analysis method to characterize the hemodynamics of the arterial segments in the COW by using arterial spin-labeling (ASL) based non-contrast-enhanced dynamic MR angiography (dMRA).
MATERIALS AND METHODS: The developed analysis method uses a graph model, bootstrap strategy, and ensemble learning methodologies to determine the time curve shift from ASL dMRA to estimate the flow direction within the COW. The performance of the method was assessed on 52 subjects, by using the flow direction, either antegrade or retrograde, derived from 3D phase-contrast MR imaging as the reference.
RESULTS: A total of 340 arterial segments in the COW were evaluated, among which 30 (8.8%) had retrograde flow according to 3D phase-contrast MRI. The ASL dMRA-based flow direction estimation has an accuracy, sensitivity, and specificity of 95.47%, 80%, and 96.34%, respectively.
CONCLUSIONS: Using ASL dMRA and the developed image analysis method to estimate the flow direction in COW is feasible. This study provides a new method to assess the hemodynamics of the COW, which could be useful for the diagnosis and study of cerebrovascular diseases.
ABBREVIATIONS:
- AcomA
- anterior communicating artery
- ASL
- arterial spin-labeling
- COW
- circle of Willis
- dMRA
- dynamic MR angiography
- PC
- phase-contrast
- PcomA
- posterior communicating artery
- TCD
- transcranial Doppler
- tMIP
- temporal dimension maximum intensity projection
SUMMARY
PREVIOUS LITERATURE:
COW is an important component of cerebral collateral circulation. The arterial structure of COW and its clinical relevance have been extensively studied by using traditional angiographic techniques such as DSA, CTA, and MRA. However, the flow in COW is less studied partially due to the limitations of the existing techniques. ASL dynamic MRA is an emerging non-contrast-enhanced technique that can provide both structural and flow information of intracranial arteries. However, its capability for estimating the flow direction in COW arteries is underexplored and the corresponding postprocessing method is yet to be developed.
KEY FINDINGS:
A novel postprocessing method to determine the blood flow direction of the COW arteries from the ASL dMRA was developed, with the accuracy rate, sensitivity, and specificity being 95.47%, 80%, and 96.34%, respectively. Retrograde flow, occurring in 8.8% of the evaluated arterial segments, were successfully detected.
KNOWLEDGE ADVANCEMENT:
This study demonstrated that ASL dMRA has great potential in estimating the flow direction of COW arteries by using a dedicated postprocessing method.
Cerebral collateral circulation plays a pivotal role in maintaining cerebral blood flow, especially when principal conduits fail, and extending the treatment window before revascularization.1 The circle of Willis (COW), which is the primary collateral circulation mechanism in the brain, bridges the interhemispheric blood flow pathways and between the anterior and posterior circulations, and can provide rapid response to cerebral blood flow alteration in acute vascular events.2 Therefore, a comprehensive evaluation of the structure and blood flow of the COW is important for the diagnosis and treatment of cerebrovascular diseases.
To date, due to the wide availability of angiography techniques, such as digital subtraction angiography,3 CT angiography,4 and MR angiography,5 the patency and caliber of the arterial segments in the COW have been extensively explored regarding their characteristics and clinical relevance.6 However, the blood hemodynamics within these segments were less studied and assessed in clinical practice, which could be largely attributed to the difficulty in its measurement. In general, the blood hemodynamics could be assessed either by monitoring the traveling of contrast agents within the vascular networks with time-resolved or artery-selective angiography techniques,7 or by directly measuring the local blood flow velocity by using techniques such as transcranial Doppler (TCD)8 and phase-contrast (PC) MR imaging.9 DSA is usable for evaluating the hemodynamics of the COW but is limited by its invasiveness. Time-resolved CTA and contrast-enhanced MRA have difficulty in offering sufficient spatial-temporal resolution to depict the small-sized COW arteries.10 While TCD provides flow direction information, visualization of the intracranial arteries is not infrequently limited by the lack of acoustic windows. PC MR imaging allows measurements of pixel-wise velocity for the COW9 but suffers from long scan time and difficulty in choosing an appropriate encoding velocity to achieve good vessel visibility and accurate flow quantification for both large and small-sized arteries.
Non-contrast-enhanced dynamic MR angiography (dMRA)–based arterial spin-labeling (ASL) is an emerging technique that can achieve both high spatial resolution (eg, 0.8 mm isotropic) and temporal resolution (up to ∼100 ms) for 4D vascular imaging.11⇓-13 It thus has great potential in characterizing the hemodynamics of intracranial arteries. Shao et al14 show that ASL dMRA can be used to quantify the arterial blood flow, arterial blood volume, arterial transit time, and mean transit time for application in arteriovenous malformation. However, the usefulness of ASL dMRA for depicting flow in the COW is yet to be explored.
Flow direction is an important feature of the COW that is helpful in identifying the vessels responsible for ischemia, the brain regions potentially in a vulnerable state and needing special attention.15 In this proof-of-concept study, we hypothesized that flow direction information within the COW can be derived from ASL dMRA. To test the hypothesis, we developed an ASL dMRA image analysis method to derive this information, and then evaluated its performance on a cohort with atherosclerotic diseases by using the velocity direction derived from 3D PC MR imaging as the reference.
MATERIALS AND METHODS
Participants
The institutional review board approved this study, and all participants gave written informed consent before recruitment. As a subcohort of the study named Carotid Intraplaque Hemorrhage: MR imaging of Therapeutic Response and Clinical Sequelae, 52 subjects with the following inclusion criteria were recruited: age ≥18; no contradictions to MR imaging; asymptomatic from carotid disease; at least 1 carotid artery had >15% stenosis. The exclusion criteria of the study were as follows: subjects with systemic inflammatory disease or atrial fibrillation; pregnant; history of bilateral carotid endarterectomy or stent placement; history of neck radiation therapy; severe chronic illness or chronic disability that will limit life span and may lead to incomplete study procedures. The subjects’ demographic characteristics are shown in Table 1.
Clinical characteristics of the enrolled subjects (n = 52)
MR Imaging Protocol
All MR imaging studies were conducted on a 3T Ingenia CX scanner (Philips) with a 32-channel array head coil. MR imaging protocol included 3D PC-MR imaging and a recently developed ASL dMRA technique, named iSNAP.11 iSNAP is a time-efficient multicontrast cerebrovascular imaging sequence that can yield ASL dMRA, static MRA, vessel wall images, and T1-weighted brain structural images simultaneously in a single scan. It consists of flow-sensitive alternating inversion recovery ASL preparations and 3D golden angle radial spoiled gradient-echo readout. To obtain ASL dMRA from the iSNAP sequence, multiple images at different delays after the ASL preparation were reconstructed from subsets of the original 3D k-space data by using the k-space weighted image contrast view-sharing reconstruction algorithm, and then subtraction of the images was performed between the ASL control and label acquisitions. Imaging parameters of the ASL dMRA with iSNAP were: turbo field echo factor 280, total number of turbo field echo shots 186, field of view 205 × 180 × 144 mm3, voxel size 0.8 × 0.8 × 0.8 mm3, TR/TE 7.6/2.7 ms, flip angle 6°, total scan time 6.5 min. The ASL dMRA was reconstructed with 20 frames with a temporal resolution of 100 ms (2s in total). Imaging parameters of 3D PC-MR imaging were: spoiled gradient recalled-echo, field of view 180 × 180 × 70 mm3, voxel size 0.5 × 0.5 × 1.0 mm3, TR/TE 13/6 ms, flip angle 12°, receiver bandwidth 96 Hz/pixel, velocity-encoding (VENC) value 100 cm/s in all 3 directions, acquisition time 5 minutes.
Tracing of COW Artery Centerlines
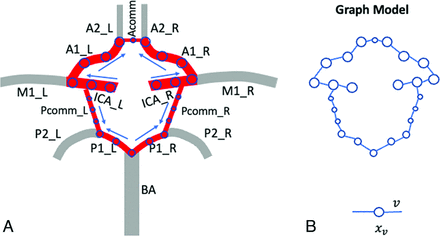
Maximum intensity projection along the temporal dimension (tMIP) was generated from the ASL dMRA (Fig 1A) and then used to trace 3D centerlines of intracranial arteries, including that of the COW, with a custom-made semi-automated intracranial artery feature extraction software named iCafe.16 A typical artery centerline extraction example is shown in Fig 1B. The traced arteries included left and right internal carotid arteries at their carotid terminus, left and right anterior cerebral arteries at their A1 segments, anterior communicating artery (AcomA), left and right posterior cerebral arteries at their P1 segments, and left and right posterior communicating arteries (PcomAs), as shown in Fig 1C. Rigid registration was applied between the magnitude image from 3D PC and T1-weighted-like images obtained from iSNAP by using SPM12 (https://www.fil.ion.ucl.ac.uk/spm). Then, the artery centerlines obtained from the ASL dMRA were translated to the co-registered 3D velocity map obtained from 3D PC-MR imaging.
A, Representative ASL dMRA images obtained by the iSNAP sequence. B, Centerline tracing results based on the tMIP of dMRA. C, The labeled COW arteries for flow direction estimation. D, Time signals along the centerline of ICA, measured from ASL dMRA. The red ones correspond to the proximal sites of ICA, while the light yellow ones correspond to the distal sites.
Flow Direction Analysis on 3D PC-MR Imaging
Then, for each arterial segment, 3 cross-sectional images that are perpendicular to the artery centerline, as obtained from tMIP of ASL dMRA, at the 1/6, 3/6, and 5/6 of the segment, were generated. Vessel lumen contours were subsequently automatically extracted by using the active contour method on the cross-sectional angiography images17 and manually corrected. Then, the flow direction in each vessel segment was determined by the average velocity vector of the 3 cross-sectional slices.
Flow Direction Analysis on iSNAP dMRA
Fig 1D shows the typical intensity time curves extracted from the ASL dMRA for the voxels along the centerline of artery. The flow direction within the COW can be predicted by estimating the time curve shift and identifying the source of the signal profile that reaches the peak earlier. It is worth noting that unlike cardiac-triggered 4D CTA or 4D flow MR imaging, the flow direction obtained from ASL dMRA is averaged across cardiac phases. Three principal methodologies, namely graph modeling,18 bootstrap strategy,19 and ensemble learning,20 were utilized to determine the time curve shift from ASL dMRA. A graph model was constructed based on the COW anatomy. The bootstrap strategy, a resampling approach in statistics and machine learning, can be used to estimate signal patterns from high-dimensional data. Ensemble learning, another technique from statistics and machine learning, combines multiple models to enhance predictive performance. The following is an in-depth explanation of each methodology.
An undirected graph model with node features 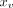 and edges
v was first built based on the morphology of the COW, as shown in Fig 2. Node features
and edges
v was first built based on the morphology of the COW, as shown in Fig 2. Node features 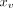 represent a point on the centerline of the arterial segment with
N time points, with
A as the symbol of the adjacency matrix, and
D as the symbol of the degree matrix. Each element of
A represents whether there is a connection between 2 nodes
represent a point on the centerline of the arterial segment with
N time points, with
A as the symbol of the adjacency matrix, and
D as the symbol of the degree matrix. Each element of
A represents whether there is a connection between 2 nodes  and
and  .
D is a diagonal matrix, where each diagonal element
.
D is a diagonal matrix, where each diagonal element  stands for the number of nodes connected to node
i. Laplace matrix
L of the graph was derived by
stands for the number of nodes connected to node
i. Laplace matrix
L of the graph was derived by
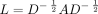
A, Predefined antegrade blood flow direction in the COW. B, The graph model built based on the COW.
By utilizing the low-pass characteristic of
L, we can get a smoother graph  after we multiply
L to the node feature matrix
X. Here smoother means features between adjacent nodes become more alike.
after we multiply
L to the node feature matrix
X. Here smoother means features between adjacent nodes become more alike.
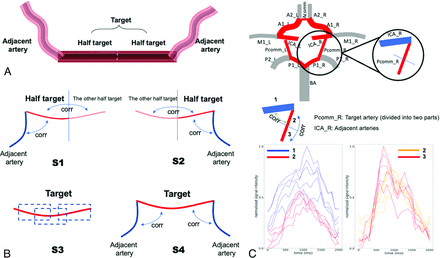
Next, the flow direction was determined by using the filtered node feature matrix X' through the combination of bootstrap strategy and ensemble learning. For each arterial segment, 4 ensembled strategies were employed to calculate the flow direction independently. These strategies focused on the correlation between different parts of the targeted arterial segment and were subsequently combined to produce the final flow direction result, as shown in Fig 3. The targeted artery was divided into halves, with each half connected to the adjacent artery (which may be absent). The flow direction in the “half target” segment was determined for the first 2 strategies, as illustrated in Figs 3B and 3C. Specifically, k pixels were randomly sampled at each time in each segment and repeated n times. The correlation between the points in each sampling from adjacent arterial segments, “half target,” and the “other half target” segment was then calculated. The 2 correlation coefficients calculated in each sampling were compared, and the flow direction was assigned “+1” for the antegrade direction and “-1” for the retrograde direction. Finally, the results were summed up, and the flow direction was saved for the final ensemble decision. For the 3rd strategy (S3), half of the points in the target artery were randomly sampled each time and repeated n times. The correlation between distance and time-to-peak was calculated. The flow direction was assigned “+1” for antegrade flow direction and “−1” for retrograde flow direction. The sum of the length n sequence was computed. The 4th strategy (S4) was similar to the first 2, but the correlation was calculated between the adjacent arterial segment and the target artery instead of dividing into halves. If 1 side of the target artery was missing, the correlation on that side was considered equal to -inf. The final flow direction of the target artery was determined by whether the total sum of all 4 length n sequences was greater than 0.
The 4 strategies employed in this study to estimate the flow direction of COW arteries based on ASL dMRA. A, Illustration of different parts of the target artery and its adjacent arteries. B, Schematics showing how the correlation coefficients calculate flow direction. In these schematics, flow direction was estimated for the deep red segment, with the shallow red segment being the other half on the same artery and the deep blue segments being the adjacent arteries. C, An example showing how to derive flow direction for the target artery (right PcomA) from strategy 1 in the COW. The correlation between the adjacent artery (right ICA, #1) and the first half of the target artery (#2) was first calculated, and then the correlation between the first half of the target artery (#2) and the other half of the target artery (#3) was calculated. The 2 plots only showed 1 sampling time with the number of sampling points equal to 5. Determination of the flow direction will depend on the sign of the sum of the correlation coefficient from Part 1–2 and Part 2–3.
Image Quality Control and Data Exclusion
On ASL dMRA, if the arterial centerline could not be clearly identified and traced by 1 experienced radiologist (>5 years) and 1 experienced medical imaging analysis reviewer (>3 years), the corresponding artery segment would be excluded from the analysis. On 3D PC, the artery segment would be excluded from the analysis if the lumen contours on all 3 cross-sectional slices could not be depicted by an automated 2D lumen contour segmentation algorithm from SPM12 and double checked by an experienced radiologist (>5 years).
Ablation Study
Ablation studies were performed to explore the individual and combined effects of the sampling time and number of sampling points on the estimation of flow direction, which will be evaluated by accuracy, sensitivity, and specificity. The sampling time ranges from 1000 to 5000, with an interval of 500. The number of sampling points ranges from 3 to 6, with an interval of 1. The number of sampling points should be at least 3, as this is the minimum number required to calculate the correlation coefficient. The accuracy, sensitivity, and specificity of the flow direction estimation were calculated for each setting.
Evaluation Metrics and Statistical Analysis
Blood flow direction was defined as antegrade or retrograde. The predefined antegrade flow direction for the COW arteries is illustrated in Fig 2A. For communicating arteries, the flow direction was not predefined for AcomA and the predefined antegrade flow direction in PcomA is the anterior circulation to posterior circulation. Any blood flow direction opposite to the direction shown in Fig 2A was considered retrograde. Segment-wise accuracy, sensitivity, and specificity of the flow direction prediction results acquired by the proposed method on ASL dMRA were calculated by using the flow directions estimated from 3D PC-MR imaging as the criterion standard.
RESULTS
Flow Direction Results from 3D PC-MR Imaging
Among the 52 subjects, 340 arterial segments in the COW were evaluated, including left and right ICA, left and right A1 and P1, AcomA, left and right PcomAs. Six of 340 (1.8%) artery segments were excluded due to low image quality of cross-sectional PC-MR imaging impeding assessment checked by an experienced radiologist (>5 years). Twenty-three of 340 (6.8%) segments were AcomA. Among the rest of the segments, 281/340 (82.6%) had antegrade flow directions, while 30/340 (8.8%) had retrograde directions including left and right A1, P1, and PcomA, according to the 3D PC-MR imaging.
Arterial Segment Summary and Flow Direction Results from ASL dMRA
The length and radius of the COW arteries measured from ASL dMRA, as well as the accuracy of flow prediction, are shown in Table 2. In 310/334 (92.8%) arterial segments, the flow directions obtained by using ASL dMRA were consistent with those by using 3D PC-MR imaging. The detailed prediction accuracy for each arterial segment is also shown in Table 2. Table 3, without including the AcomA, shows the accuracy, sensitivity, and specificity of flow direction predictions from ASL dMRA with different analysis strategies. The final accuracy, sensitivity, and specificity after combining all strategies were 92.80%, 83.33%, and 93.96%, respectively. Among the 23 AcomA arterial segments, 18/23 (78.3%) arterial segment flow directions were aligned with 3D PC-MR imaging, while 5/23 (21.7%) had wrong predictions.
Length, radius, and correct prediction counts for arterial segments (n = 52)
Accuracy, sensitivity, and specificity of the 4 strategies, along with overall performance metrics
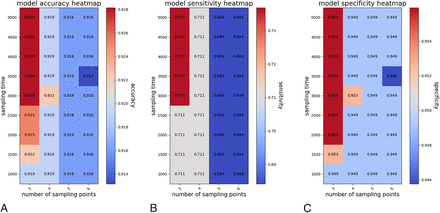
Ablation Study
The results of the ablation study are shown in Fig 4. The optimal parameter combinations were a sampling time of 3000 and a number of sampling points of 3.
Accuracy (A), sensitivity (B), and specificity (C) of the flow direction estimation in the ablation study, in which different sampling time and the number of sampling points were used.
DISCUSSION
In the current study, we demonstrate the feasibility of using ASL dMRA to evaluate the flow direction in individual arterial segments of the circle of Willis by using a graph-based bootstrap technique. Using 3D PC-MR imaging as the reference, the proposed method can predict the flow direction with high accuracy, sensitivity, and specificity.
Method Evaluation
The basic idea used in this study is to estimate the temporal shift of the signal curve from points on the arteries of the COW. The following methods were employed to improve the estimation. First, the arterial network of the COW was represented as a graph. Such representation not only improves the characterization of the inherent connection between the arteries but also serves as a low-pass filter to reduce the noise in the time signal. Similar ideas were widely used in cognition studies to represent connections of neurons or in diffusion MR imaging models to describe functional interactions in brain structure.21,22 Second, the bootstrap strategy, a powerful statistical procedure to estimate target variables, was used to lower the variance and uncertainty of the prediction result. Third, 4 different strategies were employed and combined to derive the final flow direction. This “ensemble learning” strategy has been shown to improve the predictive performance.20 Specifically, in this study, the first 2 strategies focused on the flow pattern in 2 adjacent arterial segments, the third strategy focused on the flow direction within the arterial segment, and the last strategy focused on the completeness of the circle.
Most of the arteries with an inconsistent flow direction between 3D PC and ASL dMRA are the communicating arteries. This may be attributed to their small length and lower blood flow, which not only result in a larger noise but also make artery tracing more difficult. The fact that the flow directions inside the communicating arteries may vary over time23 may also contribute to the discrepancy.
Significance and Comparison with Other Modalities
The clinical significance of the COW is largely dependent on its role in redistributing the blood flow between the different flow territories and protecting the brain tissue against potential flow deficiency caused by cerebrovascular diseases. Therefore, assessing the COW’s flow condition is more important and desirable than only assessing its morphology, although the 2 aspects are somehow related. Retrograde flow, as assessed by the proposed method, is a critical aspect of flow dynamics. Previous studies have indicated that retrograde flow is often a sign of compromised hemodynamics, which can lead to several adverse outcomes such as increased risk of ischemic events and poor tissue perfusion.24,25 Additionally, Li et al26 have discussed how retrograde flow can serve as a critical marker for assessing the severity of artery occlusions, significantly impacting clinical decision-making and prognostication. However, due to the limit of imaging techniques, many previous studies that explored the clinical relevance of the flow condition in the COW only considered its morphologic configuration.27 Therefore, developing a reliable imaging technique with good clinical acceptance and availability is important for facilitating the study and diagnosis of flow condition in the COW.
Conventional DSA is recognized as the criterion standard for assessing the COW, attributed to its superior spatiotemporal resolution and vessel selectivity.28 However, its application has been limited by the invasiveness, ionizing radiation, and higher cost as compared with the imaging modalities. Another occasionally used technique is 4D-CTA; however, current 4D-CTA techniques have limited temporal resolution to acquire an accurate flow profile in collateral circulation.29 Although 1 previous study demonstrated their ability to acquire flow direction and flow velocity by using gamma distribution extrapolation,30 further validation work on 4D-CTA is still needed. 4D-flow MR imaging is another novel quantitative state-of-the-art technique to measure cerebral blood flow profile; however, more efficient reconstruction and postprocessing methods are needed to solve the long scan time, low spatial resolution, and long processing times.31 TCD sonography is a real-time, noninvasive, and cost-effective technique for evaluating collateral circulation by providing spectral flow waveforms, flow direction, and velocity information.8 However, visualization of the intracranial arteries may be poor due to limited acoustic windows. There are several advantages for ASL dMRA, including its noninvasive, nonradioactive nature, and high spatial and temporal resolution, facilitating accurate assessment of COW blood flow. Above all, ASL dMRA demonstrated accurate quantification results compared with PC-MR imaging.
Clinical Applications
ASL dMRA has several potential clinical applications. Visualization of flow patterns could be helpful in the evaluation of collateral circulation for treatment planning when considering surgical arterial sacrifice,32 revascularization surgeries in Moyamoya disease,4 aneurysm treatment planning,33 and assessment of cerebrovascular reserve in combination with carbon dioxide challenge.34 In addition, it can also be used for the assessment of collateral flow patterns in chronic arterial occlusions,35 or assessment of potential etiologies in cryptogenic stroke,36 to determine flow patterns and potential arterial sources for emboli. Furthermore, the application of our proposed method with and without vascular stimulus could potentially identify flow redistribution patterns and detect vascular steal in steno-occlusive disease, which will be explored in future studies.37
Carotid Stenosis and Retrograde Flow
An additional analysis was performed by using the Chi-square test to compare the incidence of retrograde flow between the subjects with any large carotid stenosis (>50%) and the subjects without. A trend was observed that the group with large stenosis tended to exhibit a greater incidence of retrograde flow, but the difference was not statistically significant (45.5% versus 23.5%, P = .26). Further research with a larger sample size is needed to confirm this observation.
Limitations
There are several limitations in this study. First, the criterion standard for flow direction measurement, ie, DSA, was not available for the studied subjects. Therefore, 3D PC MR imaging was used as the reference, considering that it has been well-validated for measuring the flow in the COW.38 However, we retrospectively included 2 patients with Moyamoya disease from a pilot study who underwent both preoperative ASL dMRA and DSA imaging to support our study. The flow direction predictions from ASL dMRA by using the proposed method are consistent with the flow directions from DSA (Online Supplemental Data). Second, the centerline of AcomA could not be extracted accurately for some subjects due to a low signal intensity and short length. This may compromise the flow direction estimation from ASL dMRA and 3D PC-MR imaging. Another limitation is that the sample size is small, and only subjects with carotid atherosclerosis were included. This has limited the generalizability of the findings. Therefore, in the future, the proposed method needs to be further validated for other cerebrovascular diseases, such as intracranial atherosclerosis, cerebral aneurysm, arteriovenous shunting, etc.
CONCLUSIONS
This proof-of-concept study shows that high temporal-spatial resolution ASL dMRA combined with a dedicated postprocessing method can be used to estimate the flow direction in the COW with high accuracy. Therefore, ASL dMRA has the potential to become a useful noninvasive tool for assessing the collateral flow of the COW in cerebrovascular diseases.
Footnotes
This research has been supported by the National Heart, Lung, and Blood Institute/National Institutes of Health under grants R01 HL103609 and R01 HL103609-S1.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received April 3, 2024.
- Accepted after revision May 16, 2024.
- © 2024 by American Journal of Neuroradiology