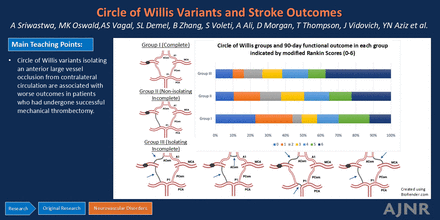
Graphical Abstract
Abstract
BACKGROUND AND PURPOSE: Leptomeningeal collaterals have been associated with better outcomes in large-vessel stroke, but little is known about how the circle of Willis (CoW) collaterals affect stroke outcomes. We aimed to determine the relationship between 3 anatomically distinct CoW subtypes and 90-day outcomes in patients with acute ischemic stroke after successful revascularization via endovascular thrombectomy (EVT).
MATERIALS AND METHODS: We performed a retrospective analysis of patients treated with successful EVT for large-vessel occlusion at a comprehensive stroke center between May 2016 and November 2023. The CoW anatomy was trichotomized by using baseline CT angiography as follows: 1) complete circle of Willis (C-CoW), 2) nonisolating incomplete circle of Willis (NI-CoW), and 3) isolating incomplete circle of Willis (I-CoW). χ2 and logistic regression analyses were utilized to determine the association of the CoW subtype with 2 coprimary outcomes: the 90-day mRS and 90-day mortality.
RESULTS: A total of 465 patients were included in the analysis. Multivariable logistic regression analysis demonstrated a significant association between I-CoW and 90-day mRS compared with NI-CoW (OR [95% CI], 1.83 [1.08–3.09]; P = .02). Additionally, I-CoW anatomy was associated with a higher 90-day mortality than C-CoW (OR [95% CI], 2.58 [1.01–6.60]; P = .04) and NI-CoW (OR [95% CI], 1.89 [1.13–3.18]; P = .01).
CONCLUSIONS: CoW variants are associated with functional and mortality outcomes in patients treated with EVT for anterior circulation large vessel occlusion. Further research is needed to determine how CoW vessel anatomy may impact clinical assessment, triage, and treatment in acute ischemic stroke.
ABBREVIATIONS:
- ACA
- anterior cerebral artery
- ACom
- anterior communicating artery
- AIS
- acute ischemic stroke
- CoW
- circle of Willis
- C-CoW
- complete circle of Willis
- EVT
- endovascular thrombectomy
- I-CoW
- isolating incomplete circle of Willis
- LVO
- large vessel occlusion
- mTICI
- modified treatment in cerebral infarction
- NI-CoW
- nonisolating incomplete circle of Willis
- PCA
- posterior cerebral artery
- PCom
- posterior communicating artery
- Tan CS
- Tan collateral scores
SUMMARY
PREVIOUS LITERATURE:
Knowledge about how circle of Willis (CoW) collaterals affect large vessel stroke outcomes is lacking. Some studies have observed functional and mortality benefits in patients having a complete CoW versus an incomplete CoW. Others have found no such benefit or have only observed benefit in a select patient population (ie, internal carotid artery occlusions) or with specific vessels that comprise the CoW (for example, posterior communicating artery). We aimed to analyze the impact of variant CoW anatomy relative to the occluded anterior large vessel on stroke outcomes in patients treated successfully with mechanical thrombectomy.
KEY FINDINGS:
Analysis of variant CoW anatomy in 465 patients with successfully revascularized anterior LVO demonstrated significant association of isolating CoW variants with higher 90-day mortality rates (P = .01) and poorer functional outcome (P = .02) compared with nonisolating CoW variants. Higher 90-day mortality was also noted when compared with the patients with complete CoW (P = .04).
KNOWLEDGE ADVANCEMENT:
In patients with successfully treated anterior circulation LVO, isolating CoW variant anatomy negatively impacts the 90-day functional and mortality outcome. This critical observation, if replicated in larger multicenter studies, may impact triage and treatment in acute ischemic stroke.
Cerebral collateral circulation is an essential variable for predicting functional outcomes in acute ischemic stroke (AIS) due to large vessel occlusion (LVO). Collateral blood flow to the ischemic penumbra is a positive predictor of smaller infarct growth, a lower risk of hemorrhagic transformation, and extended therapeutic windows.1,2
The circle of Willis (CoW) is a major source of collateral blood flow in the brain.3 The anterior communicating artery (ACom) and posterior communicating artery (PCom) of the CoW provide collateral flow between the left and right hemispheres and the anterior and posterior circulation, respectively.4 Variance in these, as well as other constituents of the CoW, are common, with estimates indicating 54%–83% of the population having an incomplete CoW due to hypoplasia or aplasia in at least 1 vessel comprising the CoW.5 For example, estimates from cadaveric studies regarding hypoplasia of the ACom range from 9%–30%, with total aplasia of the ACom in nearly 2% of the general population.6 Similarly, hypoplasia or aplasia of the PCom occurs in approximately 68% of the population.5
The impact of CoW variance on anterior LVO stroke is not completely understood. This is likely due to the mitigating effect of leptomeningeal collaterals, which are a second important source of collateralization during AIS. The leptomeningeal arteries direct blood via retrograde flow, whereas the CoW vessels supply antegrade blood flow.7 An incomplete CoW variant has been linked to higher odds of developing an AIS.8⇓-10 However, an incomplete CoW does not necessarily result in poor collateral flow in the affected hemisphere. While some studies have found that CoW completeness is associated with better 90-day functional outcomes, others have not.11⇓⇓⇓-15 These studies are limited by small sample size and subsequent inability to control for leptomeningeal collaterals. Furthermore, the effect of hypoplastic or absent CoW arteries isolating the anterior LVO-affected hemisphere from the rest of the intracranial circulation (posterior or contralateral anterior) has not been analyzed.
We aimed to determine the relationship between 3 anatomically distinct CoW subtypes and 90-day functional outcomes in patients with AIS after successful endovascular thrombectomy (EVT). The CoW subtypes were classified based on their ability to provide collateralization from the posterior and/or contralateral cerebral hemispheres. We hypothesized that CoW subtypes that do not isolate the occluded anterior large vessel and the affected cerebral hemisphere from the posterior and contralateral circulation would be correlated with better functional and mortality outcomes. This paper follows the Strengthening the Reporting of Observational Studies in Epidemiology Checklist reporting guidelines.
MATERIALS AND METHODS
Study Population
We retrospectively analyzed prospectively collected data from an institutional registry of all consecutive patients with LVO treated at a comprehensive tertiary stroke center between May 2016 and November 2023. Patients who presented with AIS stroke due to occlusion of the ICA or the M1 segment of the MCA and underwent successful EVT (defined as modified TICI [mTICI] score 2b or greater) within 24 hours of symptom onset were included.16 Patients presenting beyond 24 hours of symptom onset or last known well, those with tandem occlusions, or with mTICI 2a or lower post-EVT score, were excluded. The local LVO registry was approved under umbrella protocol #2016–6858 by the institutional review board. The requirement for informed consent was waived because of the retrospective nature of the study.
Covariates and Study Outcomes
Variables including demographics (age, sex, race), medical history (hypertension, hyperlipidemia, congestive heart failure, diabetes mellitus, atrial fibrillation, smoking, prior history of stroke), and clinical characteristics (NIHSS score, point of care blood glucose [mg/dL], IV thrombolysis administration, and time to revascularization [minutes]) were obtained from the registry. NIHSS scores were recorded at the time of the first encounter. The primary outcome of this study was functional outcome, defined by the 90-day mRS score after initial stroke symptoms or time of last known well.17 The 90-day mRS was collected by stroke study nurses. The score was dichotomized into good functional outcome (mRS 0–2; normal to mild disability with most activities of daily living being independently maintained) and poor functional outcome (mRS 3–6; moderate to severe disability, or death).
Imaging Variables
CT head reports had ASPECTS for 395 of 465 cases (85.2%) included in the analysis. For the remaining cases, 2 senior radiology residents (J.V., D.M.) and 1 neuroradiology fellow (T.T.) reviewed the CT head to determine ASPECTS. A neuroradiology fellow with an additional fellowship in neuroradiology research (A.S.) independently adjudicated all cases for accuracy. CTA head imaging was reviewed to determine the site of vascular occlusion, CoW anatomy, and Tan collateral scores (Tan CS) for all 465 cases.18 The radiology trainees reviewed the scans independently, and any disagreements in their assessments were re-evaluated. Discrepancies were handled by a board-certified neuroradiologist (L.W.). All radiologists were blinded to patient outcomes. The CoW anatomy was trichotomized by using baseline CTA of the head as follows (illustrated in Fig 1 and representative examples on CTA in the Supplemental Data):
Group I, complete circle of Willis (C-CoW): All 7 arteries that comprise the CoW are robust and well-opacified by contrast. These include the A1 segments of the anterior cerebral artery (ACA), P1 segments of the posterior cerebral artery (PCA), PCom arteries, and ACom arteries.
Group II, nonisolating incomplete circle of Willis (NI-CoW): Variants created by absent or hypoplastic (<1 mm in caliber) CoW vessels that have the potential to limit blood flow but do not isolate the occluded artery and affected cerebral hemisphere from the posterior circulation or the contralateral anterior circulation.
Group III, isolating incomplete circle of Willis (I-CoW): Variants created by absent or hypoplastic CoW vessels that isolate the occluded artery and affect the cerebral hemisphere from the posterior circulation and contralateral anterior circulation.
Three distinct anatomic groups of CoW vessels. Group I (C-CoW): all 7 CoW arteries are normally developed, as illustrated. Group II (NI-CoW): incomplete but functionally NI-CoW variants that allow communication from the contralateral anterior or posterior circulations to the occluded vessel. There are many specific variants that apply to this category. Here, we show just 1 for illustrative purposes. Group III (I-CoW): comprises cases with functionally I-CoW variants that preclude communication with the occluded vessel from contralateral anterior circulation and posterior circulation. Four examples are depicted here for illustrative purposes. The arrows in the figures represent hypoplastic or absent CoW arteries. Illustration created by using software from BioRender.com. A1 = A1 segment-ACA; P1 = P1 segment-PCA.
Tan CS in the affected cerebral hemisphere was assessed on baseline CTA brain as follows: 0, absent collateral supply; 1, supply filling >0% but ≤50%; 2, supply filling >50% but <100%; and 3, 100% collateral filling of the occluded MCA territory.18 ASPECTS, assessed for each patient on baseline CT head, and the site of vascular occlusion, assessed on CTA, were obtained from clinical neuroradiology attending interpretation if present in the electronic medical record. If not documented, the ASPECTS were read by radiology fellows (A.S. and T.T.).
The final mTICI score was obtained from the LVO registry and abstracted from the procedure notes by the neurointerventionalist. mTICI scores were classified as follows: grade 0, no perfusion; grade 1, antegrade perfusion past the initial occlusion but limited distal branch filling with little or slow distal perfusion; grade 2a, antegrade perfusion of less than one-half of the occluded target artery territory; grade 2b, antegrade perfusion of more than one-half of the occluded target artery territory; grade 2c, near complete antegrade perfusion except for slow flow or distal emboli in a few distal cortical vessels; grade 3, complete antegrade perfusion of the occluded target artery territory.19 Patients who achieved mTICI 2b or greater postrevascularization were included in the analysis.
Statistical Analysis
Descriptive analyses of patient demographics and clinical characteristics are reported as mean ± SD for age and count (percentage) for sex and medical history. The normality of the distribution of data points was assessed by using the Kolmogorov-Smirnov test. The χ2 test and univariate logistic regression models were used to evaluate the association of CoW groups with functional and mortality outcomes. Predetermined covariates associated with stroke outcomes (age, sex, baseline ASPECTS, admission NIHSS, blood glucose levels, IV thrombolytic status, symptom onset to revascularization time, and Tan CS) were then fitted into multivariable logistic regression models along with the CoW groups.20⇓⇓⇓⇓-25 All analyses were performed by using SAS Version 9.4 (SAS Institute). Statistical significance was set at P < .05.
RESULTS
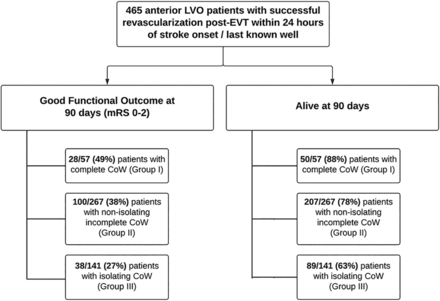
Among the 1820 patients with AIS admitted to the comprehensive stroke center between May 2016 and November 2023, 465 met all inclusion criteria without any exclusion criteria (Fig 2). The mean age (SD) of the patients was 67.0 (±15.3) years; 51% were men, and 17% were black. All collected demographic, clinical, and imaging characteristic data are provided in the Supplemental Data. A total of 57 (12%) patients demonstrated C-CoW, 267 (57%) had NI-CoW, and 141 (30%) had I-CoW. Good 90-day functional outcomes were observed in 166 (36%) patients and poor outcomes in 299 (64%) patients.
Flowchart outlining the steps of patient selection for the study.
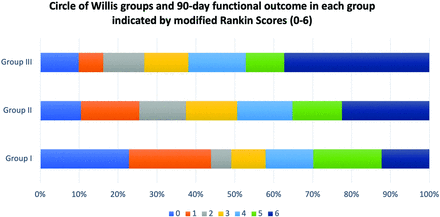
The percent rates of good functional outcomes and mortality among the 3 distinct CoW groups are shown in Fig 3. Individual mRS scores were analyzed among the 3 CoW groups, and a percentage-based trend toward worse outcomes and increased mortality was observed in the I-CoW group compared with the NI-CoW and C-CoW groups (Fig 4). The χ2 test demonstrated a significant difference in the 90-day mRS (P = .007) and mortality (P < .001) between the 3 CoW groups. Univariate logistic regression analysis demonstrated a significant association between I-CoW and poor functional outcomes when compared with the C-CoW (OR [95% CI], 2.64 [1.40–5.00]; P = .003) and NI-CoW (OR [95% CI], 1.63 [1.04–2.55]; P = .03) groups (Table).
Functional outcome and mortality at 90 days among the 3 CoW groups.
Graph demonstrating 90-day poststroke functional outcome indicated by mRS (0–6) in the 3 CoW groups, where 0 indicates no residual deficit and 6 indicates death.
Multivariable logistic regression model assessing the association of CoW groups and other covariates with 90-day functional and mortality outcomes (good, mRS 0–2, versus poor, mRS 3–6)
A multivariable logistic regression analysis was performed to assess the strength of the association after accounting for predetermined covariates (age, NIHSS, blood glucose, ASPECTS, Tan CS, and time to revascularization). A significant association of I-CoW with poor 90-day functional outcomes was again demonstrated when compared with NI-CoW (OR [95% CI], 1.83 [1.08–3.09]; P = .02) (Table). However, there was no significant difference in functional outcomes between the I-CoW and C-CoW groups (OR [95% CI], 1.87 [0.89–3.92]; P = .10).
Multivariable logistic regression models also demonstrated a significant association between I-CoW and higher 90-day mortality compared with C-CoW (OR [95% CI], 2.58 [1.01, 6.60]; P = .04) and NI-CoW (OR [95% CI], 1.89 [1.13, 3.18]; P = .01) (Table). Additionally, a comparison of the absence of residual deficit (mRS 0) among the 3 CoW groups at day 90 poststroke revealed a significant difference (P = .01) by using χ2 analysis.
We performed a post hoc analysis after adding intravenous thrombolysis administration as a covariate in the existing logistic regression model. Intravenous thrombolysis did not attain significance when compared with 90-day functional outcome (P = .35) and trended toward significance when compared with 90-day mortality (P = .06). The analysis demonstrated significant association of the I-CoW group (group III) with worse 90-day mortality compared with both the NI-CoW (OR [95% CI]: 1.93 [1.18, 3.16], P = .01) and C-CoW (OR [95% CI]: 2.68 [1.06, 6.78], P = .04) groups. The association of the I-CoW group with 90-day functional outcome, however, became borderline insignificant when compared with the NI-CoW group (OR [95% CI]: 1.93 [0.95, 2.53], P = .08) and insignificant when compared with the C-CoW group (OR [95% CI]: 1.55 [0.76, 3.18], P = .23).
Analysis of the distribution of hypoplastic and absent CoW arteries in the incomplete CoW groups (I-CoW and NI-CoW) showed that the PCom arteries were hypoplastic or absent in most cases compared with other CoW arteries (Supplemental Data). Specifically, the PComs were hypoplastic or absent in 90% (127/141) of the I-CoW group and 85% (226/267) of the NI-CoW group, although this difference was not significant (P = .34). In contrast, AComs were hypoplastic or absent in 85% (119/141) of the I-CoW group and 14% (37/267) of the NI-CoW group, showing a significant difference between the 2 groups (P < .001). These findings suggest that the ACom could be a greater determinant of collateral flow than the more commonly absent or hypoplastic PCom. The A1 segment of the ACA was hypoplastic or absent in the fewest cases, with 12% in I-CoW and 1.8% in NI-CoW (P < .001).
DISCUSSION
In successfully treated anterior circulation patients with LVO, we found that I-CoW variants were associated with lower functional independence and higher mortality than NI-CoW variants at 90 days. I-CoW variants were also associated with a higher 90-day mortality than C-CoW variants. In the univariate logistic regression model, statistical significance was achieved in favor of good functional outcomes in the C-CoW group compared with the I-CoW group. Surprisingly, this association was not maintained in multivariate analysis. The reason for this is unclear but may be due to the small sample size within the C-CoW subgroup (n = 57).
Our findings are consistent with those of previous studies examining the role of CoW variants and their effect on functional outcomes in patients with AIS. For example, 1 study found that the odds of good 90-day functional outcomes (mRS 0–2) were nearly 12 times higher in patients with an ACom or PCom. When both the ACom and PCom were present, the same study found the odds of a good functional outcome to be 29 times higher. They also concluded that AComs had a higher impact on stroke outcomes.26 Another study demonstrated that the presence of PCom is an independent predictor of survival.27 Finally, a third study concluded that the presence of an ACom in ICA occlusions leads to more favorable leptomeningeal collateralization.28 Our study is in alignment with these results as the analysis of AComs demonstrated a higher frequency (85% versus 14%) and significant association (P < .001) of absent or hypoplastic ACom with isolated CoW compared with nonisolated CoW, further underscoring the importance of ACom in collateralization.
However, there have been a few conflicting reports on the impact of specific CoW constructs on stroke outcomes. Some of these studies, like ours, were exclusively performed in patients undergoing EVT.26,28⇓-30 Others included patients without consideration of intervention.9,31 Irrespective of the inclusion criteria, most of these studies determined a significant association between CoW variants and severity of stroke, but to varying extents and qualifying conditions that may oppose our reported findings in this study. A 2023 systematic review of 11 studies determined that the CoW plays a crucial role in stroke outcomes when distal ICA occlusions are present, yet plays no role in M1 occlusions, citing a study performed by Westphal et al29 in 2021.15 However, this study acknowledges a trend toward better mortality outcomes in patients with a complete CoW, and it is possible that the sample size of the smaller subgroups of the CoW variants statistically limited the study. The overall conclusion of the systematic review agrees with our paper that the integrity of the CoW is important for stroke prognosis.15 Another recent study evaluating stroke outcomes in 182 patients found that an incomplete CoW was not associated with functional outcomes after successful endovascular therapy.14 Of note is that this study was limited by a smaller sample size compared with our investigation, and ACom patency was not included in the analysis due to concerns regarding its reliable detection on their imaging technique of choice (MRA). Despite this, the authors estimate a significant association may be reached in cohorts exceeding 3000 individuals.
Many studies concerning CoW anatomy and its correlation with long-term functional outcomes have focused on the presence of communicating arteries only or have analyzed the effect of incomplete versus complete CoW variants.9,13,14,31,32 However, we could only find 1 previous study that analyzed the impact of the functional characterization of CoWs, in patients who had undergone EVT, relative to the occluded vessel and its associated vascular territory.28 Our investigation is unique to the literature, as it provides a more comprehensive assessment of CoW anatomy to account for the potential for collateral blood flow beyond 1 vessel and includes a sample size 3 times larger than the most similarly designed study.28 Furthermore, we classified these variants into 3 subgroups to better understand the cumulative effects of specific variants on outcomes. The fact that variants that isolate the ischemia-affected cerebral parenchyma from the posterior and contralateral anterior circulations result in worse outcomes emphasizes the mitigating influence of these variants on ischemia.
For the CoW anatomy to be truly isolated, 1 or more vessels from both the anterior CoW (comprising ACom and left and right A1-ACA) and posterior CoW (comprising left and right PCom and P1-PCA) need to be hypoplastic or absent. This anatomy was observed in nearly one-third of the patients in our study population, emphasizing that it is not uncommon, further underscoring the importance of CoW anatomy in stroke outcomes in a large proportion of patients despite the best efforts at revascularization and penumbra salvage. Additionally, the study findings suggest that the ACom is a greater determinant of collateral flow than the more commonly hypoplastic or absent PCom. This observed effect of anterior CoW variants on collateral flow has been seen in another study before ours, albeit concerning the A1 segment of the ACA in the other study.33
Importantly, the interdependence of CoW anatomy and Tan CS in determining leptomeningeal collateralization, and the resultant protective effect in ischemia, has not been well-analyzed in previous studies. In our patient cohort, a significant correlation was demonstrated between good (2–3) versus poor (0–1) Tan CS and the CoW groups on pair-wise comparison. Furthermore, in the multivariable logistic regression analysis, Tan CS were significantly associated with both 90-day functional and mortality outcomes after controlling for other covariates, including CoW groups. These results underscore the combined role that these 2 important determinants of leptomeningeal collateralization play in mitigating the harmful effects of vessel occlusion.
The post hoc analysis performed with intravenous thrombolysis as an additional covariate in the existing multivariable logistic regression model demonstrated significant association of CoW anatomy with 90-day mortality. However, it failed to demonstrate a significant association with the functional outcome. This lack of association was most likely because the study was not sufficiently powered to incorporate additional covariates. Larger studies are required to validate these results.
Our study is limited by its retrospective design, lack of information regarding premorbid mRS, time to needle, final infarct volume, anatomic or other physiologic circumstances that could affect collateral blood flow (ie, severe hypotension or heart failure), and dichotomized outcome scoring of functional outcomes. Further, we did not include CTP imaging data because it is only performed routinely in a minority of patients before EVT. Finally, our rate of C-CoW is smaller than the rates reported in the literature, likely due to the numbers being derived from the general population and nonstroke patients. Our study is strengthened by a unique classification of CoW variants designed to account for potential stepwise decreases in collateral blood flow and a relatively large sample size that allowed for control of the leptomeningeal collateral score in outcome correlation.
The American Heart Association guidelines state that it may be reasonable to incorporate collateral flow status in EVT eligibility decision-making.34 Considering the established relationship between CoW anatomy and collateral capacity, CoW anatomy may be a helpful consideration when predicting the outcomes of patients with AIS. Additionally, targeted analysis evaluating their impact on large core infarcts needs to be performed.
CONCLUSIONS
CoW variants are associated with functional and mortality outcomes in patients treated with EVT for anterior large vessel occlusion. Further research is needed to determine how CoW vessel anatomy may impact the clinical assessment, triage, and treatment of AIS.
Footnotes
This work was supported by the NINDS NeuroNEXT grant and by American Heart Association Grant https://doi.org/10.58275/AHA.24CDA1268532.pc.gr.193580
Aakanksha Sriwastwa and Michael K. Oswald are co-first authors of this manuscript.
Yasmin N. Aziz and Lily Li-Li Wang are co-last authors of this manuscript.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received August 14, 2024.
- Accepted after revision December 19, 2024.
- © 2025 by American Journal of Neuroradiology