Graphical Abstract
Abstract
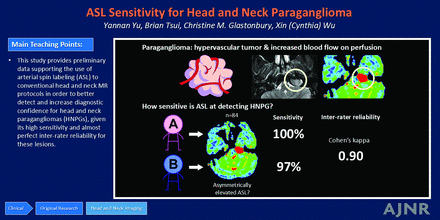
BACKGROUND AND PURPOSE: Head and neck paragangliomas (HNPGs) are rare neuroendocrine tumors whose hypervascular nature allows differentiation from many other head and neck neoplasms. We aimed to investigate the sensitivity of arterial spin-labeling (ASL) MR sequences for the detection of HNPGs.
MATERIALS AND METHODS: All head and neck MR examinations performed at a single tertiary institution between 2015 and 2023 were searched. Studies using ASL sequences that indicated either clinical suspicion for or ultimate imaging diagnosis of HNPG were identified. These studies were independently reviewed by 2 neuroradiologists blinded to the original radiology reports to determine, in a stepwise fashion, the following: 1) whether there was asymmetrically elevated blood flow on ASL imaging, 2) whether ASL findings correlated with lesions identifiable on conventional anatomic images, and 3) whether lesions likely reflected paragangliomas on the basis of correlations with clinical, laboratory, pathology, and other radiology data (Disagreement between raters was resolved by consensus.). The Cohen κ coefficient and the sensitivity of ASL in identifying HNPGs were calculated.
RESULTS: Eighty-four patients were included in the analysis (mean age, 54 [SD, 14] years and 47 women). Thirty patients had lesions confirmed or found likely to be HNPG, and 54 patients had lesions found unlikely to be HNPG or had no identifiable lesion. Among 46 of 84 patients with ASL blood flow asymmetry, 43 (93%) had lesions correlated with a lesion identifiable on anatomic imaging. Asymmetrically elevated ASL blood flow that correlated with a lesion demonstrated a sensitivity of 100% for reader A and 97% for reader B for identifying HNPG. The Cohen κ coefficient was 0.90 (SD, 0.11) between the 2 readers (P < .001). Among 18 cases with pathology- or dotatate PET–proved HNPG, the sensitivity was 100% for reader A and 94% for reader B.
CONCLUSIONS: Asymmetrically elevated blood flow on ASL imaging demonstrates high sensitivity for the detection of HNPG, with almost perfect interrater agreement.
ABBREVIATIONS:
- ASL
- arterial spin-labeling
- HN
- head and neck
- HNPG
- head and neck paraganglioma
SUMMARY
PREVIOUS LITERATURE:
HNPGs are rare neuroendocrine tumors that demonstrate hypervascularity and hyperperfusion. Previous studies have shown that MRA and dynamic contrast-enhanced MR perfusion can help differentiate between HNPGs and other HN masses. ASL, a perfusion method that is relatively resistant to susceptibility artifacts present in the HN region, may offer added diagnostic value in the evaluation of HNPGs without needing IV contrast, but its sensitivity for doing so remains underexplored.
KEY FINDINGS:
Asymmetrically elevated ASL blood flow demonstrated 97%–100% sensitivity and 74% specificity for diagnosing HNPGs with a Cohen κ coefficient of 0.90, indicating almost perfect interrater reliability. ASL in addition to conventional MR sequences had a sensitivity of 100% and specificity of 93% in diagnosing HNPGs.
KNOWLEDGE ADVANCEMENT:
This study provides preliminary data supporting the use of ASL in conventional head and neck MR protocols to better detect and increase the diagnostic confidence for HNPGs, given its high sensitivity and almost perfect interrater reliability for these lesions.
Paragangliomas are rare hypervascular neuroendocrine tumors arising from sympathetic or parasympathetic paraganglia. Sympathetic paragangliomas typically secrete catecholamines and usually arise from the paravertebral ganglia of the chest, abdomen, and pelvis. Rarely, functional paragangliomas can occur in the head and neck (HN). In contrast, parasympathetic paragangliomas do not secrete catecholamines (ie, They are nonfunctional.). They are mostly located along the vagal and glossopharyngeal nerves in the HN, arising at the carotid body, nodose ganglion of the vagus nerve, jugular bulb, tympanic plexus, and larynx.1,2
Imaging is a crucial component of diagnostic assessment of paragangliomas. A 68Ga/64Cu-dotatate scan is considered the most accurate imaging technique3,4 in detecting paraganglioma, but the cost and availability of dotatate are variable.5 Contrast-enhanced MRI and CT are more commonly used as the first-line imaging technique for suspected HN tumors, with MRI benefitting from higher soft-tissue contrast resolution in the neck.
Compared with most other HN masses, head and neck paraganglioma (HNPG) demonstrates hypervascularity and hyperperfusion. On CT, arterial or early venous phase hyperenhancement is a valuable distinguishing feature of HNPG. Similarly, studies have shown that MR TOF angiography and MR perfusion can differentiate paragangliomas and other HN masses such as meningiomas, schwannomas, and metastases.6⇓-8
In contrast to conventional dynamic contrast-enhanced or dynamic-susceptibility contrast MR perfusion, both of which require IV gadolinium contrast, the arterial spin-labeling (ASL) technique is a noninvasive MR perfusion technique. ASL uses radiofrequency-labeled arterial protons in the entry arteries as contrast material and estimates the transit delay from the labeled vessel to the tissue of interest.9,10 This transit delay provides a relatively accurate estimation of blood flow without the administration of contrast.11,12 Due to its ease of implementation, ASL is used in many HN and brain protocols at our institution.
However, to our knowledge, the sensitivity of ASL to detect HNPGs has not been established. We aimed to investigate the interrater reliability, sensitivity, and specificity of ASL to detect HNPGs in patients with suspected and incidental paragangliomas.
MATERIALS AND METHODS
A Health Insurance Portability and Accountability Act–compliant, institutional review board–approved search of all radiologic reports of MRIs of the HN (including the brain, skull base, internal auditory canals, face, and neck) dated between 2015 and 2023 from a single institution was conducted with the following search terms: “glomus” or “jugulare” or “jugulotympanicum” or “paraganglioma” or “carotid body.” These search terms were used to identify examinations that were ordered with the clinical indication for paraganglioma and those with an imaging diagnosis of paraganglioma, even if the diagnosis was not initially clinically suspected. MRI was obtained with 3T MR from multiple manufacturers and models, including Magnetom Vida (Siemens), Ingenia (Philips Healthcare), Signa Premier (GE Healthcare), Signa HDxt (GE Healthcare), and Discovery MR750 (GE Healthcare). Protocols included MR brain, MR internal auditory canal, MR face, and MR neck. ASL sequence parameters are as follows: 3D pseudocontinuous ASL with labeling time = 2000 ms, postlabeling delay = 2.0 seconds, and labeling plane offset = 2 cm; TE = 22 ms, TR = 4800 ms, inversion time = 3820 ms, number of averages = 1, flip angle = 120°, slice thickness = 4 mm, matrix size = 64 × 64, bandwidth = 3005 Hz/px.
From the initial search results, studies without ASL acquisitions were excluded. The remaining cases were independently reviewed by 2 neuroradiologists (reader A with 12 years of experience, reader B with 5 years of experience) blinded to the original radiology reports. The presence or absence of asymmetrically elevated ASL blood flow was recorded while the neuroradiologists were blinded to other sequences and clinical information. “Asymmetric ASL blood flow” was defined as appreciable solid-appearing ASL blood flow asymmetry larger than the voxel size variation and higher than adjacent cerebral cortical blood flow. Conventional image sequences, including pre- and postcontrast T1-weighted imaging, T2 fat-suppressed imaging, or T2-weighted FLAIR and DWI, were then reviewed to determine whether ASL findings correlated with lesions identifiable on anatomic images. Finally, the readers made final diagnoses on whether the lesions likely reflected paragangliomas on the basis of all available clinical data, including the results of other imaging modalities such as dotatate PET scans and surgical pathology, when available. Disagreement between the 2 readers was resolved with a consensus review.
Relevant clinical information was obtained through chart review, including age, sex, MR study protocol, MR study indication, pathology and dotatate PET data, history of hereditary paraganglioma syndrome or high-risk mutation carrier status, and paraganglioma location and sidedness. Statistical analyses were conducted in STATA (Stata/MP 18; StataCorp). Continuous data were expressed as mean (SD), and categoric data were expressed as a counts with percentages. The Cohen κ coefficient was used to evaluate interrater reliability.13 The χ2 or Fisher exact test and independent-samples t test were used for comparisons of clinical data when appropriate. The sensitivity and specificity of identifying paraganglioma using asymmetrically elevated ASL blood flow were calculated in all patients and several subgroups, including patients with dotatate PET and/or pathology-confirmed paragangliomas, patients with pathology or other criterion standard tests proving nonparaganglioma, and studies with and without an indication of paraganglioma. We obtained the exact 95% CIs for sensitivity and specificity from the binomial distribution.
The methodology proposed by the Standards for Reporting of Diagnostic Accuracy Studies (STARD) checklist was followed.
RESULTS
The initial search yielded studies from 193 patients, from which 109 (109/193, 56%) patients were excluded due to lack of the ASL acquisition. Eighty-four (84/193, 44%) patients were therefore included in the study, with a mean age of 54 (SD, 14) years, 47 (47/84, 56%) of whom were women.
Thirty patients (30/84, 36%) had lesions confirmed or considered likely to be HNPG, of whom 15 (50%) had pathologic confirmation, and 3 (10%) had dotatate PET confirmation alongside an underlying high-risk genetic predisposition for paraganglioma formation. The remaining 12 (40%) patients in this cohort were considered likely to have HNPGs based on clinical and imaging information. Twenty-three patients (23/30, 77%) had unilateral HNPGs, and 7 (7/30, 23%) had bilateral lesions.
Twenty-five patients (25/84, 30%) had lesions considered unlikely to be paragangliomas, 12 of which (12/25, 48%) were proved nonparaganglioma by either pathology or other criterion standard examinations, including angiography-confirmed dural arteriovenous fistula (n = 1), pathology-proved hemangioma (n = 1), schwannoma (n = 4), meningioma (n = 2), epithelial neoplasm (n = 2), basal cell adenoma (n = 1), and osteomyelitis (n = 1). Twenty-nine patients (29/84, 35%) had no identifiable lesion. Demographic and clinical data are shown in Table 1.
Patient demographic and clinical data
Both readers identified asymmetrically elevated ASL blood flow in 46 of the 84 cases (46/84, 55%). Of these 46 cases, reader A found that 44 (44/46, 96%) and reader B found that 43 cases (43/46, 94%) had a correlation with lesions on anatomic imaging. The Cohen κ coefficient was 0.90 (SD, 0.11) (P < .001) between the 2 readers for any asymmetrically elevated ASL blood flow and 0.88 (SD, 00.11) (P < .001) for asymmetrically elevated ASL blood flow that correlated with a lesion, accepted to reflect almost perfect agreement.13 There were 4 cases in which readers disagreed on ASL asymmetry, but they both agreed independently on whether the lesions were likely paragangliomas. The single case on which the 2 readers disagreed on the diagnosis was a subcentimeter right skull base lesion with associated ASL blood flow elevation and enhancement in a patient who had pathologically proved meningioma with hyperperfusion. The disagreement was resolved by consensus, and both readers agreed that this was not likely a paraganglioma. Unfortunately, there was no pathologic data to further characterize the lesion.
Asymmetrically elevated blood flow on ASL imaging that correlated with a lesion demonstrated a sensitivity of 100% (95% CI, 100%–100%) and specificity of 74% (95% CI, 61%–84%) for reader A and 97% (95% CI, 79%–100%) and 74% (95% CI, 61%–84%) for reader B for identifying paragangliomas (Tables 2 and 3).
Elevated ASL blood flow in detecting paraganglioma
Among 18 cases with pathology- or dotatate-proved paraganglioma, the sensitivity was 100% (18/18; 95% CI, 100%–100%) for reader A and 94% (17/18; 95% CI, 65%–99%) for reader B. Among 15 cases with pathology-proved paraganglioma, the sensitivity was 100% (15/15; 95% CI, 100%–100%) for reader A and 93% (14/15; 95% CI, 58%–99%) for reader B. Among 12 cases with proved nonparaganglioma by pathology or other criterion standard examinations, reader A found 7 (58%) cases and reader B found 8 (67%) cases with asymmetric ASL blood flow. Cases with no lesions, only 1–2 cases (4%–7%, readers A and B, respectively) were rated as having asymmetrically-elevated ASL blood flow. Examples of true-positive, true-negative, and false-positive cases are shown in Figs 1–3.
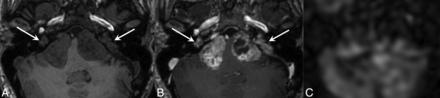
Pathology-proved left jugular paraganglioma with ASL blood flow elevation. A female patient in her 50s initially presented with left-sided pulsatile tinnitus and was found to have an SDHD mutation. Surgical resection of a left middle ear mass revealed paraganglioma. Preoperative T1WI precontrast (A) and T1WI postcontrast (B) MR demonstrated an enhancing mass in the left jugular foramen extending into the left middle ear (thick arrow) and a smaller right jugular foramen mass (thin arrow). C, ASL blood flow map shows marked elevated blood flow associated with left jugular mass (thick arrow) and mildly elevated blood flow associated with right jugular mass (thin arrow). Surgical resection of the left middle ear portion of the mass revealed paraganglioma. The right-sided jugular foramen mass was not biopsied but was assumed to also represent paraganglioma, given the patient’s underlying genetic mutation.
Pathology-proved nonparaganglioma (schwannoma) without ASL blood flow elevation. A female patient in her 30s with a history of neurofibromatosis type 2 with bilateral cerebellopontine angle masses. The patient underwent subtotal resection of a left-sided mass, which revealed a schwannoma, followed by radiation therapy. Preoperative axial T1WI precontrast (A) and T1WI postcontrast (B) MR shows bilateral cerebellopontine angle heterogeneously enhancing masses (white arrows). C, While mild signal heterogeneity was observed at the jugular foramen, the ASL blood flow map shows no significant solid blood flow elevation associated with the masses.
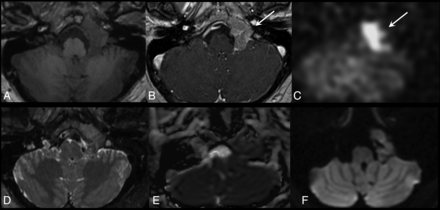
Pathology-proved nonparaganglioma with associated ASL blood flow elevation. A male patient in his 40s initially presented with a headache. Preoperative T1WI precontrast (A) and T1WI postcontrast (B) MR shows an enhancing mass with osseous remodeling. C, ASL blood flow map shows associated blood flow elevation (white arrows). D, T2WI shows that the mass demonstrates signal isointensity to cerebellar gray matter. ADC (E) and DWI (F) demonstrate mild reduced diffusion. The initial clinical diagnosis was paraganglioma, while surgical pathology revealed meningioma.
For studies with suspected paraganglioma or surveillance of known paraganglioma history, reader A had a sensitivity of 100% (24/24; 95% CI, 100%–100%) and specificity of 92% (12/13; 95% CI, 59%–99%) and reader B had a sensitivity of 96% (23/24; 95% CI, 74%–99%) and specificity of 85% (11/13; 95% CI, 54%–96%) in this selected cohort. For studies with all other indications, both reader A and reader B detected asymmetrically elevated ASL blood flow in all incidental paragangliomas (6/6, 100%).
Using the consensus read as the reference, the clinical radiology report at the time of the MR examination demonstrated 100% (30/30; 95% CI, 100%–100%) sensitivity and 93% (50/54; 95% CI, 89%–100%) specificity for identification of paragangliomas (Table 3).
Sensitivity and specificity of ASL and clinical radiology reports
DISCUSSION
This retrospective study demonstrated that asymmetrically elevated ASL blood flow in the skull base or neck had a sensitivity of 97%–100% and specificity of 74% in detecting HNPGs in this selected cohort. Asymmetrically elevated ASL blood flow also demonstrated almost perfect interrater reliability between 2 neuroradiologists with 12 and 5 years of experience. This study provides preliminary data to support the use of ASL to detect HNPGs.
Most HNPGs are parasympathetic, and <4% may demonstrate sympathetic function, secreting catecholamines.1,14 Symptoms of a nonfunctional HNPG mostly depend on its anatomic locations, given its slow growth.15 Carotid body paragangliomas, located at carotid bifurcations, are often asymptomatic until they become large masses, inducing cranial nerve dysfunction or presenting as a neck mass. Although they have a low malignant potential,16 they are preferably treated with surgical resection. Vagal paragangliomas located along the course of the vagal nerve may present with various symptoms, including pulsatile tinnitus, cranial nerve deficits such as hoarseness (X), dysphagia (IX), shoulder drop (XI), aspiration, and hemiatrophy of the tongue (XII). These tumors may also be asymptomatic. Radiosurgical treatment is often preferred, given the high risk for vagal nerve injury, with an open approach. Tympanic paragangliomas located in the middle ear along the Jacobsen nerve induce pulsatile tinnitus and hearing loss. Jugular paragangliomas, centered at the jugular foramen in the skull base, can occlude venous outflow and induce pulsatile tinnitus, hearing loss, and dizziness. Jugular paragangliomas can also cause cranial nerve deficits when they grow or invade the adjacent cranial nerves.
While HNPGs can arise sporadically, a considerable portion of them are associated with genetic syndromes and high-risk mutations,14 which accounted for 53% of our cohort. Accurate and timely diagnosis of paragangliomas would allow appropriate management, including genetic testing, functional imaging or biopsy, and surgical or radiation treatment, especially in symptomatic cases or cases with tumor growth.1,17 For asymptomatic or indolent HNPGs in high-risk locations such as the skull base,1 the diagnosis is often achieved with imaging features alone and tumors managed with active surveillance and radiation therapy, because surgical risks may outweigh the benefit. Therefore, only a limited number of the patients in our cohort had pathologic data to support their HNPG diagnosis.
Contrast-enhanced MR angiograms may add diagnostic value to conventional MR sequences in identifying HNPG, with a reported sensitivity of 100% and specificity of 94% in a small cohort study of 27 patients.7 MR perfusion can assess blood flow and may aid in differentiating paragangliomas from nonparaganglioma tumors.1,18 Among common skull base lesions, paragangliomas demonstrate significantly higher ASL perfusion compared with other hypervascular lesions such as meningiomas and metastases, except for hemangioblastomas, which demonstrate similar hyperperfusion.6
Multicenter prospective studies with a cohort of 238 cases have shown that conventional MR and contrast-enhanced MR angiograms have a sensitivity of 90%–95% and specificity of 92%–99% in diagnosing HNPGs.19 However, the MR angiogram interpretation of HN lesions may be more complicated than perfusion imaging, with a reported interrater reliability of 0.67–0.77.7 In comparison, our single-center data showed an almost perfect interrater reliability of 0.90.
In our cohort, the clinical radiology report of conventional MR plus ASL imaging at the time of the study had a sensitivity of 100% and specificity of 93% in diagnosing HNPGs, which is like the reported sensitivity and specificity of contrast-enhanced MR angiography, though direct comparison is not allowed, given different cohorts and institutions. With ASL blood flow alone, the sensitivity of detecting paraganglioma is high (97%–100%), indicating that ASL is very useful in excluding the diagnostic possibility of HNPGs. However, ASL blood flow elevation is not particularly specific in diagnosing paraganglioma, because other common skull base lesions such as meningiomas, hemangiomas, and osseous metastases may also demonstrate hyperperfusion.6 Therefore, despite its high sensitivity for HNPGs, we do not recommend using ASL alone for the diagnosis of HN tumors. Instead, its value lies in helping increase the sensitivity and diagnostic confidence of radiologists as they synthesize all available conventional imaging and clinical information in the assessment of potential HNPGs.
The study has several limitations. First, some of the cases had no pathology or dotatate PET confirmation of paraganglioma diagnosis due to various practical reasons, such as small lesion size and preference for conservative management. However, each case was analyzed using all available imaging and clinical information and follow-up data to maximize diagnostic accuracy. Second, the examinations were performed on a variety of MR scanners from different manufacturers. Therefore, parameters for ASL and other sequence acquisitions were not unified across all examinations. However, this variability reflects the real-world practice of most large hospital systems that use multiple scanner systems. The high interrater reliability and sensitivity achieved despite this variability speaks to the value of the ASL technique in detecting HNPGs. Third, although we have blinded the readers to the clinical and final diagnosis of each case, they were nonetheless aware of the selective nature of the study and the study cohort, which was enriched for patients with HNPGs. Therefore, they may have been subconsciously biased toward diagnosing HNPGs, and more real-world validation in the future would be valuable to conclusively establish the high sensitivity found in this study. Fourth, the ASL sequence was qualitatively evaluated in this study. A quantitative method may provide more diagnostic value and may be investigated in future studies.
Finally, this was a retrospective study targeting MR studies that were performed for the evaluation of suspected paragangliomas or that had patients with a diagnosis of paragangliomas, enriching the data set with a higher prevalence of paragangliomas than in the general population. This highly selective cohort did not include all patients with head and neck masses that would demonstrate elevated perfusion; as a result, the reported specificity value of ASL asymmetry in identifying HNPGs may not apply to a more generalized, global cohort. Therefore, caution should be used when interpreting the specificity in this study. In addition, this study cannot provide positive predictive values or negative predictive values of ASL in the evaluation of paragangliomas to reflect real-world data. However, given that asymmetric elevation of ASL blood flow had a near-100% sensitivity for paragangliomas, the presence of this finding should raise a high suspicion for an interpreting radiologist even if no abnormality was initially identifiable on conventional imaging.
CONCLUSIONS
We demonstrated that asymmetrically elevated ASL blood flow has a high sensitivity and almost perfect interrater reliability in identifying HNPGs in a single-center cohort with suspected or diagnosed paragangliomas. This study provides preliminary data to support the use of ASL to detect HNPGs. However, ASL blood flow elevation was not particularly specific for HNPGs, and conventional imaging and clinical data should be considered when making a final diagnosis. In the future, larger studies are warranted for further validation.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
REFERENCES
- Received October 29, 2024.
- Accepted after revision January 11, 2025.
- © 2025 by American Journal of Neuroradiology