SUMMARY:
The hypoperfusion intensity ratio (HIR) is a quantitative metric used in vascular occlusion imaging to evaluate the extent of brain tissue at risk due to hypoperfusion. Defined as the ratio of tissue volume with a time-to-maximum (Tmax) of >10 seconds to that of >6 seconds, HIR assists in differentiating between the salvageable penumbra and the irreversibly injured core infarct. This review explores the role of HIR in assessing clinical outcomes and guiding treatment strategies, including mechanical thrombectomy and thrombolytic therapy, for patients with large-vessel occlusions (LVOs). Evidence suggests that higher HIR values are associated with worse clinical outcomes, indicating more severe tissue damage and reduced potential for salvage through reperfusion. Additionally, HIR demonstrates predictive accuracy regarding infarct growth, collateral flow, and the risk of reperfusion hemorrhage. It has shown superiority over traditional metrics, such as core infarct volume, in predicting functional outcomes. HIR offers valuable insights for risk stratification and treatment planning in patients with LVOs and distal medium vessel occlusions. Incorporating HIR into clinical practice enhances patient care by improving decision-making processes, promoting timely interventions, and optimizing postintervention management to minimize complications and improve recovery outcomes.
ABBREVIATIONS:
- AIS
- acute ischemic stroke
- aOR
- adjusted OR
- ASITN-CVS
- American Society of Interventional and Therapeutic Neuroradiology Collateral Score
- BMI
- body mass index
- DEFUSE 3
- Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke
- DMVO
- distal medium-vessel occlusion
- EVT
- endovascular thrombectomy
- HIR
- hypoperfusion intensity ratio
- IQR
- interquartile range
- LAMS
- Los Angeles Motor Scale
- LVO
- large-vessel occlusion
- MT
- mechanical thrombectomy
- PH
- parenchymal hematoma
- Tmax
- time-to-maximum
The hypoperfusion intensity ratio (HIR), defined as the ratio of the volume of tissue with a time-to-maximum (Tmax) of 0.10 seconds divided by the volume of tissue with a Tmax of 0.6 seconds, is a quantitative metric utilized in vascular occlusion imaging.1 It evaluates the amount of brain tissue vulnerable to hypoperfusion or decreased blood flow in proportion to the core infarct or area of permanent damage. Quantitatively, the severity of hypoperfusion is expressed numerically by HIR. This aids in determining the amount of surrounding infarct tissue that may be salvaged by intervention. HIR aids in the distinction between the penumbra and the core infarct (irreversibly injured tissue), directing treatment choices such as mechanical thrombectomy (MT) or thrombolytic therapy. It aids in identifying the penumbra, which is the region of the hypoperfused area that is salvageable. Higher HIR readings have been linked to worse outcomes for patients who have had strokes, according to research, suggesting more tissue damage and possibly less benefit from reperfusion therapy. Compared with subjective CTA collateral score systems, it may be easily interpreted and quickly obtained as an automated and quantitative instrument. HIR, derived from perfusion imaging, may be used to predict the rate of collateral flow, the pace at which the infarct grows, and the clinical outcome after endovascular therapy.2 In this review, we aim to assess the role of HIR, its safety, and clinical outcomes through previously published literature.3,4
PATHOPHYSIOLOGY OF VESSEL OCCLUSIONS
Stroke is a neurologic disorder caused by impaired perfusion through the brain’s vasculature. Blood is supplied to the brain anteriorly by 2 internal carotid arteries and posteriorly by vertebral arteries, forming a circle of Willis (Fig 1). Ischemic stroke is caused by impaired blood or oxygen supply. Approximately 85% of all stroke casualties are caused by ischemia and 15% by hemorrhage.5
Circle of Willis.
Ischemic stroke may result from either thrombotic or embolic occlusions. Thrombosis is caused by atherosclerotic narrowing of the arteries that supply the brain. At the same time, an embolic stroke is caused due to abrupt blockage of blood flow to the brain after embolus. The decreased blood or oxygen supply causes cell death, resulting in loss of neuron function. Other significant factors contributing to stroke pathology include inflammation, energy depletion, homeostatic imbalance, acidosis, elevated intracellular calcium levels, excitotoxicity, free radical–induced damage, cytokine-induced cell toxicity, complement system activation, blood-brain barrier disruption, glial cell activation, oxidative stress, and leukocyte infiltration.6⇓⇓⇓-10 The area surrounding the dead region of the brain typically has a decreased blood supply. That intact but hypoperfused area of the brain lies within the functional impairment threshold and can still recover if the blood supply is improved. The “at risk” brain tissue immediately outside the infarct core is known as ischemic penumbra. In the ischemic penumbra, cells remain viable for a short period, receiving limited blood flow from the collateral arteries of the blocked vascular tree. In this context, collateral circulation is crucial for maintaining viable brain tissue over extended periods and enabling the administration of reperfusion therapies at later stages.11
Shortly after its introduction, the ischemic penumbra became the primary diagnostic and therapeutic target for ischemic stroke.12
Endovascular thrombectomy (EVT) is now considered the standard treatment for eligible patients experiencing acute ischemic stroke (AIS) due to large-vessel occlusion (LVO), even up to 24 hours from the onset of symptoms.13 In patients with AIS due to LVO, robust collateral status, absence of prior stroke, and absence of diabetes are associated with achieving excellent recanalization after MT.14 Hence, the effectiveness of collateral blood supply to the affected hemisphere in LVO AIS significantly predicts infarct size and growth, patient functional outcomes, and the risk of hemorrhagic transformation.15,16 Consequently, collateral status can influence eligibility for EVT and the decision to transfer patients to EVT-capable hospitals.17
The duration of the brain tissue’s transition from ischemic penumbra to irreversibly infarcted core varies significantly. Collateral status plays a critical role in determining the speed of this transition. Changes in cerebral perfusion pressure during patient evaluation, transfer, and treatment (such as induction/intubation) can also influence collateral circulation dynamics.11,18
HIR
The HIR is a critical metric in assessing collateral circulation in patients with AIS. Recent research has shown that a lower body mass index (BMI) and older age are associated with poorer collateral status as measured by the hypoperfusion index, which is closely related to HIR. Specifically, the study found that lower BMI and advanced age are linked to diminished collateral circulation due to factors like the progressive loss of collateral vessels and increased arterial tortuosity with aging. This could be attributed to the smaller artery diameters and less vascular remodeling ability and compromised endothelial function, which is necessary for blood vessel expansion and dilation in response to ischemia. Furthermore, inadequate nutrition linked to a reduced BMI deteriorates vascular health even more, making it harder to sustain collateral circulation during an ischemic episode.19,20 Conversely, obesity might offer some protective effects by influencing cerebral blood flow and inflammatory responses. These insights underscore the importance of considering individual patient characteristics when interpreting HIR values, as they can significantly influence the assessment of collateral circulation and, consequently, the management and treatment strategies for patients with AIS. Understanding how BMI and age affect HIR can lead to more tailored and effective approaches in evaluating stroke severity and guiding therapeutic interventions.21
HIR has shown a weak but significant negative correlation with the American Society of Interventional and Therapeutic Neuroradiology Collateral Score (ASITN-CS), indicating that higher HIR values are associated with poorer collateral status. This relationship emphasizes the role of HIR in assessing the likelihood of successful revascularization and guiding treatment decisions. Although the compensation index was found to have a stronger correlation with ASITN-CS, the inclusion of HIR in the evaluation process remains valuable due to its ability to provide a more nuanced understanding of the ischemic environment and its impact on patient outcomes.22
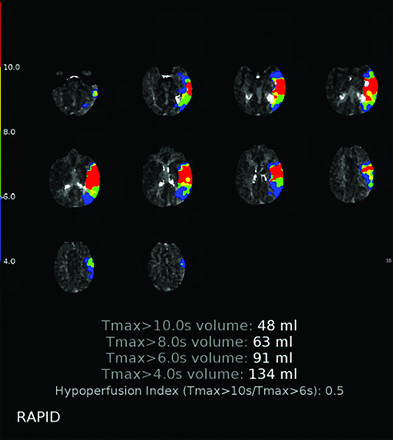
CTP is a commonly utilized neuroimaging method in assessing LVO AIS. Derived maps from CTP can estimate the extent of the established infarct core in relation to the volume of hypoperfused brain tissue at risk of infarction, often referred to as the penumbra.23,24 A commonly used parameter for estimating infarct core volume and penumbra tissue is the time at which the residue function reaches its peak or Tmax. The HIR, calculated as the ratio of tissue volume with a Tmax >10 seconds to the volume with a Tmax >6 seconds, can be quantitatively derived from CTP data sets. Tmax >6 seconds indicates brain regions where blood takes more than 6 seconds to reach tissue. Although these areas are underperfused and susceptible to ischemia, they may still be salvageable if blood supply is restored. In contrast, Tmax >10 seconds denotes regions where blood delivery takes longer than 10 seconds, indicating more severe ischemic tissue and a greater risk of permanent damage.25⇓-27 For example, in 1 scenario, the hypoperfused brain tissue volume with Tmax exceeding 6 seconds is 100 mL, while the volume with Tmax exceeding 10 seconds is 40 mL. The HIR can be calculated as HIR = 40 mL/100 mL = 0.4, meaning that 40% of the tissue is significantly hypoperfused, which can help determine treatment options such as thrombolysis or thrombectomy. Figure 2 illustrates the HIR calculation in a patient with a left MCA stroke, derived from the ratio of the volume of brain tissue with a Tmax delay >10 seconds (coded in red, 48 mL) to the volume with a Tmax delay >6 seconds (coded in green, 91 mL), yielding an HIR of 0.5. This calculation is typically performed by using automated software.
This figure illustrates the calculation of HIR in a patient with left MCA stroke. It is derived by taking the ratio of the volume of brain tissue with a Tmax delay >10 seconds (coded in red, 48 mL in this example) to the volume of brain tissue with a Tmax delay >6 seconds (coded in green, 91 mL in this example), yielding an HIR of 0.5. This calculation is generally conducted by using automated software.
HIR helps identify the portion of brain tissue that has been more severely affected by reduced blood flow in cases of ischemic infarcts. It also best predicts final infarct volume in those who are unsuccessfully recanalized.28 Though ASPECTS score, DWI, and other stroke volume measures might offer a useful baseline estimate, HIR can be more helpful in situations of failed recanalization in anticipating the infarct’s growth due to continued hypoperfusion. Even in cases in which additional problems such as distal embolism, reperfusion injury, or vascular damage occur, it can assist in understanding the likely course of the stroke by calculating the amount of tissue that is beyond salvaging. This makes it a useful predictor; nonetheless, to offer the best accurate prediction, it should be combined with other clinical and procedural data, much like any imaging biomarker.13,29 Favorable HIR profiles are associated with strong tissue collateral status, smaller initial core volumes, larger penumbra volumes, and positive functional outcomes after successful thrombectomy.30⇓⇓-33
HIR serves as a potential objective indicator of collateral status and may function as a substitute for CTA scoring methods.1,25,31,32 Regions with Tmax >6 seconds indicate varying degrees of hypoperfusion lesions, while regions with Tmax >10 seconds represent areas of severe hypoperfusion. In these areas, brain tissue lacks collateral flow and may quickly become an irreversible infarct core without timely reperfusion treatment. A higher HIR indicates a larger proportion of regions with Tmax >10 seconds.
Here, we present several cases demonstrating higher HIR with worse functional outcomes. We have an example of a 78 year old man with AIS that occurred within 9 hours who did not receive IV alteplase treatment. His baseline NIHSS score was 21, and his ASPECTS score on NCCT was 6. NCCT images (Fig 3A) revealed a left MCA hyperattenuated, gray-white matter differentiation loss within the frontotemporal region, and occlusion of the proximal left M1 segment of the MCA (red arrow). The Tmax map (Fig 3D) indicated a severe hypoperfusion area (130 mL) and a hypoperfusion area (208 mL), resulting in a high HIR of 0.6. The patient underwent MT, with pre- (Fig 3F) and post- (Fig 3G) MT images showing left M1 occlusion and postprocedural Modified treatment in cerebral infarction (mTICI) 3 after 1 pass. Follow-up DWI (Fig 3H, -I) and SWI (Fig 3J) revealed a left MCA territory infarction with hemorrhagic conversion of the left basal ganglia component.
High HIR. Baseline NCCT, CTA, CTP, DSA, and follow-up DWI and SWI in a 78-year-old man with AIS within 9 hours after onset who did not receive IV alteplase treatment. The time from onset to CT scan was 10 hours. The baseline NIHSS score was 21, and the ASPECTS on NCCT was 6. A, NCCT image shows a left MCA hyperattenuated sign and gray-white matter differentiation loss within the frontotemporal region. Axial MIP of the CTA of the head, reformatted at 16 mm, shows occlusion of the proximal left M1 segment of MCA (red arrow) (B), a more cranial slice shows collateral supply filling is greater than 0% but ≤50% of the occluded MCA territory (red arrow) (C), compatible with a Tan score of 1. D, Perfusion maps show Tmax >6 seconds, which encompasses the salvageable tissue as well as infarcted tissue, with a volume of 208 mL, and CBF <30% volume, indicative of the core infarct, of 57 mL. E, Tmax map shows the severe hypoperfusion area (red) defined Tmax >10 seconds was 130 mL and Tmax >6 hypoperfusion area (light green) was 208 mL, while HIR (= 0.6) was high. Patient underwent MT, pre- (F) and post- (G) MT images show left M1 occlusion and postprocedural mTICI 3 after 1 pass. Follow-up DWI (H and I) and (J) SWI showed left MCA territory infarct, in the left temporal lobe and basal ganglia, with hemorrhagic conversion of the left basal ganglia component.
Additionally, we encountered a case in which a patient with high HIR received appropriate management but had similar outcomes. A 76-year-old man with AIS within 2.5 hours received IV alteplase. His baseline NIHSS score was 18, and his ASPECTS score on NCCT was 6. NCCT images (Fig 4A) revealed proper MCA territory gray-white matter differentiation loss with sulcal effacement. CTA (Fig 4B) showed occlusion of the proximal right M1 segment of the MCA (red arrow). The Tmax map (Fig 4E) indicated a severe hypoperfusion area (112 mL) and (Fig 4E) a hypoperfusion area (179 mL), resulting in a high HIR of 0.6. The patient underwent MT, with pre- (Fig 4F) and post- (Fig 4G) procedure DSA images showing resultant 2b after 1 pass. Follow-up DWI (Fig 4H) and SWI (Fig 4I) revealed MCA lower division territory with petechial microhemorrhages within the infarct territories and a final infarct volume of 266 mL on DWI. The patient died 10 days later, with an mRS score of 6, indicating an unfavorable outcome.
High HIR with big final infarct volume even with short last known well (LKW). Baseline NCCT, CTA, CTP, DSA, and follow-up DWI and SWI in a 76-year-old man with AIS presenting within 2.5 hours after onset and who received IV alteplase treatment. The baseline NIHSS score was 18 and the ASPECTS on NCCT was 6. A, NCCT image shows right MCA territory gray-white matter differentiation loss with sulcal effacement. B, Axial MIP of the CTA of the head, reformatted at 16 mm, shows occlusion of the proximal right M1 segment of MCA (red arrow) (B), a more cranial slice shows collateral supply filling ≤50% of the occluded MCA territory (red arrow) (C), compatible with a Tan score of 1. D, Perfusion maps show Tmax >6 seconds which encompasses the salvageable tissue as well as infarcted tissue, with a volume of 179 mL, and CBF <30% volume, indicative of the core infarct, of 61 mL. E, Tmax map shows the severe hypoperfusion area (red) defined Tmax >10 seconds was 112 mL and Tmax >6 hypoperfusion area (light green) was 179 mL, while HIR (= 0.6) was high. Patient underwent MT, pre- (F) and post- (G) procedure DSA show resultant mTICI 2b after 1 pass. Follow-up DWI (H) and SWI (I) showed MCA lower division territory with petechial microhemorrhages within the infarct territories with a final infarct volume of 266 mL on DWI. The patient died 10 days later, with a mRS score of 6 (unfavorable functional outcome).
Generally, a high HIR (>0.4) predicts faster growth of the ischemic core, potentially limiting the effectiveness of reperfusion therapies.34 A large extent of regions with Tmax >10 seconds has been shown to correlate with poor outcomes after reperfusion. It has been evidenced by many studies that HIR moderately negatively correlates with the modified CTA collateral score, highlighting the potential challenges in managing patients with high HIR.31,35,36
A lower HIR indicates a smaller proportion of severely hypoperfused brain tissue and robust collateral blood flow in the ischemic region. Favorable collateral blood flow delays infarct progression and is associated with slower growth of the infarct, smaller final infarct volume, and better functional outcomes, instilling a sense of hope and optimism in the potential for positive patient outcomes.
This could be depicted by the case of a 63-year-old man with AIS who presented within 4 hours after onset and did not receive IV alteplase treatment. His baseline NIHSS score was 9, and his ASPECTS score on NCCT was 10. NCCT (Fig 5A) showed a hyperattenuated left MCA sign. The coronal MIP of the CTA of the head (Fig 5B) revealed occlusion of the distal left M1 segment of the MCA (red arrow). The Tmax map (Fig 5E) showed a severe hypoperfusion area (25 mL) and a hypoperfusion area (97 mL), with a low HIR of 0.3. The patient underwent MT. Pre- (Fig 5E) and post- (Fig 5F) procedure DSA images showed resultant 2c after 2 passes. Follow-up DWI (Fig 5G) showed diffusion restriction within the anterolateral left frontal lobe and anterior left temporal lobe, with confluent abnormalities in the left basal ganglia/corpus striatum, all within the left MCA territory, with a final infarct volume of 71 mL. At 90 days, the patient’s mRS was 1, indicating a remarkably favorable functional outcome.
Low HIR with good functional outcome. Baseline CTA, CTP, DSA, and follow-up DWI and SWI in a 63-year-old man with AIS presenting within 4 hours after onset and who did not receive IV alteplase treatment. The baseline NIHSS score was 9 and the ASPECTS on NCCT was 10. A, NCCT showed hyperattenuated left MCA sign (B). Axial MIP of the CTA of the head, reformatted at 16 mm, shows occlusion of distal left M1 segment of MCA (red arrow), a more cranial slice (C) shows 100% collateral supply of the occluded MCA territory (red arrow) compatible with a Tan score of 3. D, Perfusion maps show Tmax >6 seconds which encompasses the salvageable tissue as well as infarcted tissue, with a volume of 97 mL and a CBF <30% volume, indicative of the core infarct, of 0 mL. E, Tmax map shows the severe hypoperfusion area (red) defined Tmax >10 seconds was 25 mL and Tmax >6 seconds hypoperfusion area (light green) was 79 mL, while HIR (= 0.3) was low. Patient underwent MT, pre- (F) and post- (G) procedure DSA show resultant mTICI 2c after 2 passes. Follow-up DWI (G) showed diffusion restriction within the anterolateral left frontal lobe and anterior left temporal lobe with confluent abnormalities in the left basal ganglia/corpus striatum, all within the left MCA territory with a final infarct volume of 71 mL. Ninety-day mRS of this patient was 1 (favorable functional outcome).
Conversely, a higher HIR reflects a larger proportion of severely hypoperfused brain tissue, indicating poor collateral circulation in the ischemic region. This situation leads to faster infarct progression, enlargement of the infarct volume, and poorer functional outcomes.25
The HIR can be acquired within minutes after imaging by using validated automated techniques and formulated methods. This is particularly true when utilizing image analysis tools (eg, RAPID, iSchemaView; Olea Sphere, Olea Medical) that provide maps for the tissue at risk after calculating perfusion parameters like Tmax.25,37,38 While appropriate imaging methods, software quality control procedures, and an understanding of potential artifacts are necessary to ensure the validity of HIR evaluation, these considerations do not diminish the significance of HIR as a potential tool for guiding treatment.
There is disagreement regarding the necessity of utilizing CTP in AIS imaging. Some argue that native CT and CTA alone are adequate. Critics of CTP cite studies demonstrating no discernible increase in outcomes; however, this perspective overlooks the primary advantage of CTP: the ability to identify salvageable tissue (ischemic penumbra) for prompt intervention. While NCCT and CTA can reveal large infarcts, occlusions, and bleeding, they provide limited information on tissue viability, making it challenging to differentiate the ischemic core from the penumbra. For instance, the Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention with Trevo (DAWN) trial assesses clinical mismatch in the triage of wake-up and late-presenting strokes undergoing neurointervention by using DWI or CTP assessment. This study showed that thrombectomy might be beneficial for patients up to 24 hours after the onset of symptoms if they have a small ischemic core, which can be detected by using advanced imaging techniques like CTP. Significant gains in functional outcomes were observed in the trial for individuals selected based on perfusion imaging. Similarly, the Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke (DEFUSE 3) trial found that patients chosen based on advanced perfusion imaging—including CTP—performed better when thrombectomy was administered up to 16 hours after the stroke began. This trial demonstrated the crucial role of perfusion imaging in extending the therapeutic window and enhancing patient outcomes.29
LVO
Characteristics and Specific Considerations of LVO in Stroke
LVOs (ICA, MCA [M1 segment], basilar artery, and vertebral arteries) are responsible for a considerable portion of the morbidity and mortality associated with ischemic strokes. Of all AISs reported, it is estimated that at least 13%–52% are LVOs (Fig 6).21 Differences in the scaling and classification system contribute to a broader variability in prevalence.
Baseline frontal DSA projection of the right internal carotid artery demonstrating complete occlusion of the proximal right MCA M1 segment and retrograde filling of distal MCA branches from ACA-MCA pial communications. Baseline lateral DSA projection demonstrates retrograde filling of distal MCA branches from ACA-MCA pial communications.
The clinical presentation of LVO in ischemic stroke is often dramatic and characterized by the sudden onset of severe neurologic deficits.39 Acute changes in consciousness ranging from drowsiness to coma can occur, particularly with occlusions in the basilar artery or large infarcts involving the frontal lobes. With posterior circulation LVOs, dysarthria, ataxia, and vertigo can occur.40
CTA, MRA, and DSA are key modalities utilized for visualizing and assessing the occluded vessels.41 CTA is often the first-line imaging technique due to its rapid acquisition and wide availability. MRA offers high-resolution images without ionizing radiation, by using magnetic fields and radio waves to visualize blood vessels. TOF-MRA is commonly used to detect LVOs, providing clear images of the arterial system.42 DSA is considered the standard for diagnosing LVOs due to its superior spatial resolution and ability to provide real-time dynamic images of the cerebral vasculature.41
Without timely reperfusion therapy, the natural course of LVO is progressive ischemic injury and infarction of brain tissue distal to the occlusion. Mortality rates are even higher for untreated LVO, particularly with occlusions in critical vessels like the basilar artery, where brainstem infarctions can be fatal.43 Effective and timely intervention, primarily through reperfusion therapies such as IV thrombolysis and MT, has been shown to improve outcomes substantially.44 However, these therapies are not without risks, making the consideration of safety measures paramount.
Thrombolytic therapy promotes blood flow by dissolving damaging intravascular clots to prevent ischemic harm. While there are multiple side effects of thrombolytic therapy, bleeding remains a major concern. Older adults, uncontrolled hypertension, recent stroke or surgery, bleeding diathesis, and concomitant anticoagulant usage are probabilities linked to disastrous hemorrhagic consequences.45
MT is an interventional procedure commonly used in AIS. With new research demonstrating the effectiveness and superiority of MT over IV thrombolytics, the procedure’s indications have recently expanded dramatically. Stenosis at the thrombectomy site, dislodgement of a clot, vessel perforation, and intracranial bleeding are some of the complications of the procedure.45,46
Safety Outcomes
Patient Stratification.
HIR is a valuable tool in selecting patients likely to benefit from reperfusion therapies. HIR quantifies the extent of hypoperfused brain tissue, providing a detailed map of tissue viability, hence helping clinicians identify patients with salvageable brain tissue. Traditionally, IV thrombolysis is administered within 4.5 hours of stroke onset and MT within 6–24 hours.45,46
A retrospective cohort study was conducted to analyze and determine whether HIR can be used to assess patients’ eligibility for MT. The study, consisting of 197 subjects, found a favorable HIR (<0.4) strongly correlated with the decision to perform thrombectomy.32
Poor Collaterals.
Cerebrovascular collaterals have been reliably identified by HIR, with a higher value linked to rapid advancement of the ischemic core.14,21 While CTA can be used to calculate collateral scores directly, the approach has a number of disadvantages. First, the procedure is subjective. Moreover, it is rater-dependent, making it essential to invest resources in training the raters to reduce the interrater variabilities.47
Wang et al48 evaluated the relationship between the collateral score from modified CTA and the HIR via Pearson correlation in individuals with LVO. Compared with individuals with poor collaterals, those with good collaterals had a lower HIR (0.51 ± 0.2 versus 0.73 ± 0.13, P < .001) and a smaller core volume (37.3 ± 24.7 versus 116.5 ± 70 mL, P < .001). Pearson correlation revealed a relationship between a higher HIR and a lower collateral score. (P < .001, r = −0.64). The optimum HIR value for predicting a good collateral score was 0.68.48
Another study by Guenego et al31 predicted the same result in 98 patients. HIR and DSA collaterals had a significant correlation (−0.327; 95% CI: −0.494 to −0.138; P = .01). Adequate DSA collaterals were best predicted by an HIR cutoff of <0.4, with an OR of 4.3 (specificity, 0.560; sensitivity, 0.792).31 According to the ROC analysis by Olivot et al,25 an HIR >0.4 could predict poor collateral flow. While further information and investigation are required to examine the cutoff and its variations thoroughly, this could be the result of various software packages’ specified parameters and image-processing algorithms.25
The study by Lakhani et al49 found that in cases of LVO, the admission Los Angeles Motor Scale (LAMS) is independently associated with the HIR, suggesting that LAMS may serve as an indirect marker of collateral status.
Infarct Progress and Growth.
Seners et al50 demonstrated that a low HIR was linked to smaller final infarct volumes and slower infarct progression. They included 1127 patients in their study and concluded that regardless of the imaging technique, the main component linked to rapid infarct progression was HIR. Abidi et al51 examined the factors contributing to progression in infarct rates. HIR exhibited a significant correlation with the advancement rates, increasing from 27 patients (4%) in the first quartile to 527 patients (77%) in the fourth quartile.51 The multisite analysis by Koneru et al52 suggested that favorable short-term neurologic recovery is significantly correlated with relative cerebral blood flow volumes in the early time window.
This relationship between HIR and infarct progression is further exemplified in Fig 4, where we present a comprehensive case study of a 76-year-old patient with AIS. The patient, who exhibited a high HIR of 0.6, showed rapid infarct growth despite receiving IV alteplase and undergoing MT. The imaging sequence depicted in the figure illustrates the baseline infarct characteristics, including severe hypoperfusion areas, as well as the subsequent procedural outcomes. Ultimately, this led to a poor prognosis with a final infarct volume of 266 mL.
Functional Outcomes
Several factors have been recognized as crucial in forecasting positive functional outcomes among patients with AIS resulting from LVO. These factors include smaller ischemic cores, good recanalization, younger age, and less severe strokes. This approach offers a new method for outcome prediction by combining clinical factors with pretreatment imaging parameters, helping to improve early discharge planning and the effective allocation of resources. A multifaceted brain imaging technique, when applied alongside clinical evaluations, can enhance patient management and recovery, according to the study’s findings.53
Malignant Edema.
Malignant cerebral edema is a potential consequence in individuals who present with anterior circulation AIS from a major vascular occlusion (LVO) with significant infarct volumes.
In a multivariate logistic regression, age, HIR, and core infarct volume were determined to be significantly related to malignant cerebral edema. According to ROC analysis, HIR outperformed core infarct volume as a radiologic predictor of malignant cerebral edema. With an appropriate diagnostic performance cutoff of 0.54 (Youden index 0.519), the results for MCE were 14.7.54
Reperfusion Hemorrhage.
Hemorrhage, specifically parenchymal hematoma (PH), is a significant complication after endovascular treatment. A retrospective multicenter cohort study involving 624 patients reported that patients who developed PH had significantly higher HIR on admission compared with those who did not develop PH (median 0.6 versus 0.4; P < .001).55
Importantly, both higher HIR and the presence of PH on follow-up imaging were independently associated with lower odds of achieving a good clinical outcome, defined as a 90-day mRS score of 0–2 (adjusted OR [aOR], 0.83 [0.75–0.92]; P < .001 for HIR, and aOR, 0.39 [0.18–0.80]; P = .013 for PH).55
mRS
The mRS has been a standard for years for assessing stroke outcomes. It can capture the entire spectrum of functional states and score the patient’s impairment from 0 (no symptoms) to 6 (death).56
In a recent study patients with a favorable functional outcome had significantly lower HIR (0.1 [interquartile range {IQR}, 0.1–0.2] versus 0.4 [IQR, 0.4–0.5]) and higher multiphase CTA collateral scores (3 [IQR, 3–4] versus 3 [IQR, 2–3]; P < .001) compared with those with an unfavorable outcome. The HIR was strongly positively correlated with functional outcomes (r = 0.852; 95% CI: 0.813–0.884; P < .0001).56
Olivot et al25 demonstrated that at 90 days, 42 patients (42%) had a favorable functional result (mRS ≤2). Their median HIR was lower than the patients with an mRS ≥3 at 90 days. After adjustments for age, baseline DWI volume, time to MR, and early reperfusion, a low HIR was related with a 4.3 odds ratio (95% CI, 1.3–14.1), P = .016, for a favorable outcome. On the other hand, PWI lesion volume and HIR significantly interacted with functional outcomes. These outcomes were in line with Murray et al,54 where poor mRS (mRS 3–6) at 3 months was associated with larger HIR and core infarct volume and in multivariate logistic regression, only HIR (odds ratio 7.6 95% CI: 2.0–13.1) was connected with 3-month mRS.
DISTAL MEDIUM-VESSEL OCCLUSION
Distal medium-vessel occlusions (DMVOs) involve blockages in smaller branches of major cerebral arteries (M2–M4 of the MCA, anterior cerebral artery, posterior cerebral artery, and cerebellar arteries). These vessels are smaller, longer, and more fragile than large proximal vessels, making DMVOs a significant cause of AIS, leading to disability and mortality if untreated. While MT has been successful in treating proximal LVOs, its use in DMVOs is challenging due to anatomic complexity.57,58
Recent advancements in MT with newer, more navigable devices have shown promise in treating DMVOs. Early studies suggest that this approach is safe and effective, though large-scale clinical trials are needed for definitive guidelines.57 Improvements are more significant in distal than proximal lesions, though IV fibrinolysis is less effective for DMVOs. Linking EVT with DMVOs may offer better treatment outcomes due to advancements in endovascular equipment, but the heterogeneity of DMVO-related deficits complicates clinical trial design.
A study by Ozkara et al59 used machine learning to predict short-term outcomes in 69 patients with AIS with DMVOs, admitted between January 1, 2017, and September 1, 2022. The XGBoost algorithm performed best, with an Area Under the Receiver Operating Characteristic curve (AUROC) of 0.894 and an Area Under the Precision-Recall Curve (AUPRC) of 0.756. Key predictors included mismatch volume, Tmax >6 seconds, and DWI volume, suggesting that machine learning models can assist in prognosis and treatment planning for patients with DMVO.59
Koneru et al60 identified 3 critical risk factors for bleeding post-MT in medium-vessel occlusions: low cerebral blood volume index, diabetes, and more distal occlusions. These factors likely reduce brain resistance, increasing hemorrhagic risks. However, this study was limited by its small sample size and focus on the MCA, necessitating further research in other vessel locations.60
The HIR is an essential biomarker in AIS management, linked to infarct growth and outcomes. High HIR correlates with poor collateral circulation, faster infarction expansion, and worse outcomes, while low HIR predicts better outcomes.25,61⇓-63 In DMVOs, HIR is particularly valuable for assessing EVT suitability when traditional imaging is inconclusive, helping clinicians develop targeted treatment plans and improving outcomes based on collateral status.25,62,63
HIR IN COMPARISON WITH OTHER MODALITIES
The use of HIR in stroke imaging has emerged as a valuable tool in assessing cerebral ischemia, yet its reliability and effectiveness should be compared with other advanced imaging modalities such as DWI, MRI, and DSA. While HIR provides insights into collateral circulation and perfusion deficits, studies have shown that DWI is often utilized to delineate the ischemic core. However, recent evidence suggests that DWI may not solely reflect irreversible infarction, because approximately 24% of patients exhibit partial or complete reversal of DWI lesions, challenging the notion that hyperintense regions represent irreversible tissue damage. This highlights the need for a critical evaluation of the accuracy of DWI as a surrogate marker for the ischemic core, especially when compared with the more nuanced understanding provided by HIR, which considers collateral status and its implications for infarct growth and patient outcomes.64
In addition, MRI offers several advantages over DWI, particularly with the incorporation of SWI and T2*-weighted sequences, which can enhance the detection of hyperacute intracerebral hemorrhage. The use of MRI has been shown to be more sensitive in identifying smaller hematomas without mass effect compared with CT scans, potentially leading to earlier and more accurate interventions in acute stroke scenarios.65 Moreover, the ability of MRI to detect changes in tissue state over time, including the spontaneous recovery of ischemic tissue, adds another layer of complexity to the imaging landscape, suggesting that while HIR is essential in evaluating collateral status, it may benefit from complementary modalities like MRI for a comprehensive assessment of patients with stroke.
DSA also plays a crucial role in stroke management by providing detailed vascular assessments and identifying potential therapeutic targets. The safety and efficacy of DSA have been demonstrated in a developing country context, where a 0% complication rate for neurologic deficits was reported, underscoring its reliability in diverse patient populations. This contrasts with the limitations of HIR, which may not fully capture the dynamic nature of collateral circulation and its impact on infarct volumes, as observed in the DEFUSE 3 trial. This study emphasizes that collateral status can evolve over time, influencing infarct growth and highlighting the importance of integrating various imaging modalities to inform clinical decision-making effectively.66,67
HYPOPERFUSION INDICES MEASUREMENT TECHNIQUES
HIR measurement techniques are a crucial consideration that can significantly impact the reliability and reproducibility of HIR as a clinical tool in stroke management. Different perfusion imaging protocols and the use of various software algorithms can lead to discrepancies in HIR calculations. For instance, studies have shown that differences in imaging parameters, such as the timing of scans poststroke and the selection of specific thresholds for ischemic tissue, can yield varying HIR results. This variability can complicate clinical decision-making, particularly in determining the eligibility of patients for interventions such as thrombolysis or mechanical thrombectomy, as the interpretation of HIR is heavily influenced by the imaging technique utilized.67 Moreover, the interpretation of HIR measurements is not standardized across different clinical settings, which can lead to inconsistencies in diagnosing and managing ischemic strokes. For example, while some studies utilize a single-time-point assessment of Tmax to estimate HIR, others may adopt more complex models that account for dynamic changes in collateral circulation over time. This lack of uniformity in methodologic approaches can result in significant variations in patient outcomes, particularly in cases in which collateral circulation plays a pivotal role in protecting against infarct expansion.66
Furthermore, the reliance on different imaging modalities, such as DWI and MRI, adds another layer of complexity to the evaluation of HIR. For instance, while DWI is often regarded as a standard for identifying the ischemic core, recent evidence suggests that it may not be a definitive marker due to the phenomenon of DWI lesion reversal in a significant percentage of patients. This inconsistency underscores the necessity of integrating HIR assessments with other imaging modalities to achieve a comprehensive understanding of ischemic stroke pathology.64 Addressing the methodologic variability in HIR measurement techniques is essential for enhancing the clinical utility of HIR in stroke care. Standardizing perfusion imaging protocols and establishing consensus on the interpretation of HIR will improve reproducibility and reliability, ultimately leading to more informed clinical decision-making and improved patient outcomes across diverse clinical settings. This multifaceted approach will ensure that HIR serves as a robust tool in the evolving landscape of stroke management.65
LIMITATIONS, FUTURE DIRECTIONS, AND IMPLICATIONS
Despite its promise, HIR faces challenges that need addressing. The accuracy of HIR depends not only on the quality of perfusion imaging but also on the software used for its calculation, as different platforms (eg, RAPID, Olea Sphere, iSchemaView, syngo.via) may yield variations in Tmax measurements and other key parameters. This software dependency introduces potential variability in HIR results. Hence, while HIR offers valuable prognostic insights, it should be combined with other clinical data for a comprehensive assessment. Future research should refine HIR methodologies, validate its usefulness across diverse populations, and incorporate them into stroke management guidelines.25,62,63 Moreover, the software used for CTP tends to be less accurate in evaluating the posterior circulation compared with the anterior circulation.
This review underscores the importance of improving DMVO identification and treatment, highlighting the role of HIR in enhancing prognosis. While MT has been applied to DMVOs, more extensive studies are needed to evaluate its efficacy. IV fibrinolytics, though useful for distal occlusions, remain suboptimal and need enhancement. Integrating HIR into stroke management holds promise for better outcomes by objectively assessing collateral circulation’s protective capacity. Addressing these challenges could significantly advance AIS treatment.25,57,62,63,68⇓-70
For future developments, incorporating HIR into clinical practice offers potential for improved stroke treatment, especially for DMVOs. As imaging technology advances, the reliability of HIR will improve, offering better patient prognostication and treatment guidance. However, standardizing HIR measures across health care settings is crucial. Future research should focus on complex multicenter trials to demonstrate the ability of HIR to guide patient selection for treatments like MT. This approach could lead to individualized interventions that improve recovery and reduce disability in patients with stroke.25,62,63 The integration of HIR into clinical practice presents several challenges that must be addressed to enhance its utility in stroke management. A significant limitation is the technological constraints associated with imaging modalities used to derive HIR. Different imaging techniques, such as DWI and various perfusion-weighted imaging approaches, often exhibit variability in their sensitivity and specificity for detecting ischemic tissue. These differences can lead to inconsistent HIR values and complicate clinical interpretations, making it essential to develop standardized protocols that are universally applicable across diverse health care settings.64,66 Furthermore, the lack of consensus on optimal HIR measurement techniques and interpretation significantly hampers its clinical adoption. Variations in perfusion imaging protocols, software algorithms, and interpretation criteria can lead to significant discrepancies in HIR values, as evidenced by studies highlighting the role of collateral circulation and its impact on imaging outcomes. This variability raises questions about the reproducibility and reliability of HIR as a clinical tool and underscores the need for more robust validation studies across different populations and clinical contexts.65,67 Moreover, the current literature reveals a predominance of lower-level evidence regarding the application of HIR in diverse patient populations, indicating a critical gap in understanding how various demographic and clinical factors influence HIR measurements and their predictive value. Future research should aim to incorporate larger, multicenter studies that include diverse patient demographics to enhance the generalizability of HIR findings.66 In addition, further exploration of the relationship between HIR and other emerging biomarkers or imaging modalities is necessary to develop a more comprehensive assessment of patients with ischemic stroke. This approach will enable clinicians to refine their diagnostic strategies and therapeutic interventions, ultimately improving patient outcomes.
CONCLUSIONS
LVOs, including blockages in major arteries like the ICA, MCA, vertebral, or basilar artery, are critical in ischemic stroke morbidity and mortality. Without prompt treatment, these occlusions lead to severe brain ischemia and neurologic deficits. Advanced diagnostics such as CTA, MRA, and DSA are vital for early intervention.
The HIR represents a significant advancement in LVO and DMVO care, measuring brain hypoperfusion to identify patients suitable for reperfusion. HIR has superior predictive capabilities for ischemic injury and hemorrhagic complications compared with traditional metrics. Integrating HIR into clinical practice enhances risk stratification, treatment planning, and outcomes, with lower HIR values correlating with better recovery.
The collected research consistently highlights HIR as a valid measure of functional outcomes, with lower HIR values correlating with better clinical results and higher mRS values during follow-up assessments. Thus, the integration of HIR into the management of vessel occlusions significantly enhances the safety and efficacy of clinical outcomes, ultimately improving patient care and recovery prospects.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received August 20, 2024.
- Accepted after revision October 24, 2024.
- © 2025 by American Journal of Neuroradiology