This article requires a subscription to view the full text. If you have a subscription you may use the login form below to view the article. Access to this article can also be purchased.
Graphical Abstract
Abstract
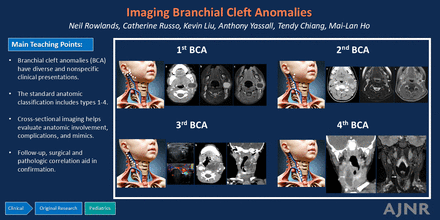
BACKGROUND AND PURPOSE: Branchial cleft anomalies (BCA) are a group of relatively common congenital pediatric neck masses, which exhibit wide variability in clinical presentation and treatment approaches. This study attempts to fill this gap by evaluating BCA clinical, radiographic, and treatment features in a large cross-sectional cohort.
MATERIALS AND METHODS: We performed a retrospective cross-sectional study of patients with BCA presenting to a single pediatric quaternary care center between 2017 and 2023. The radiology information system was queried for the term “branchial,” with diagnostic confirmation based on radiology and medical records review including patient demographics, clinical presentation and symptoms, genetic testing, interventions, and pathology. Relevant statistical tests were performed.
RESULTS: We retrospectively identified 302 unique patients with 412 imaging examinations that included the term “branchial” in the radiology information system between 2017 and 2023. The final cohort included 167 patients with a total of 246 imaging examinations. Among patients with BCA, median age at presentation was 3.3 years (range 0–22.9). Leading clinical presentations included a neck mass (88%, 147) and skin drainage (29%, 29). BCA classification was first in 37% (61), second in 44% (73), third in 4% (7), and fourth in 15% (26). Interventions included incision and drainage in 70% (121) and complete excision in 54% (91). Among patients with resected BCA, 22% (20/91) experienced at least 1 recurrence.
CONCLUSIONS: BCA have diverse clinical manifestations for which imaging aids in localization, classification, and interventional planning.
ABBREVIATIONS:
- BCA
- branchial cleft anomalies
- FS
- fat suppression
- US
- ultrasound
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
- © 2025 by American Journal of Neuroradiology
ASNR members
Login to the site using your ASNR member credentials