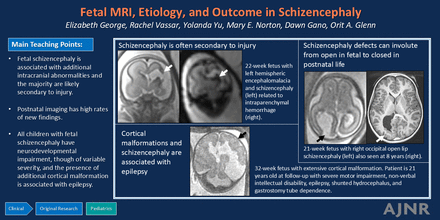
Graphical Abstract
Abstract
BACKGROUND AND PURPOSE: Schizencephaly is a rare brain anomaly that is increasingly detected in utero. There are limited data on the etiology and outcomes in fetal schizencephaly to guide work-up and counseling. We aimed to determine the associated imaging findings, etiology, and outcomes in schizencephaly detected in utero.
MATERIALS AND METHODS: This retrospective cohort study included 22 fetuses with a total of 34 schizencephaly defects identified by keyword search of fetal MRI reports from 1996 to 2022 followed by image review. Follow-up fetal and postnatal imaging, when available, was reviewed. Data on demographics, etiology, and outcomes were extracted from the electronic medical record.
RESULTS: The schizencephaly defect was open in 28/34, most common in the MCA territory (23/34), and commonly involved the frontal lobe (16/34). Additional intracranial abnormalities were seen in all fetuses, including other cortical malformations (13/22), abnormal posterior fossa (12/22), abnormal corpus callosum (10/20), and intraparenchymal hemorrhage (9/22). The cause of schizencephaly was classified as secondary (as evidenced by intraparenchymal hemorrhage at schizencephaly, monochorionic twin gestation, infection, or maternal/placental risk factor) in 64% (14/22), potentially genetic in 9% (2/22), and unknown in 27% (6/22). Among those liveborn (n = 8), we observed the following outcomes: postnatal death (1/8), tube feeding (1/7), shunted hydrocephalus (1/7), and epilepsy (4/7). Among those older than 1 year of age, cerebral palsy (4/5) and speech delay or intellectual disability (3/5) were common. Cortical malformations remote from schizencephaly were associated with epilepsy (P = .03). On postnatal imaging, open defects were often involuted (8/11), and there were high rates of new/additional findings (4/6).
CONCLUSIONS: In this cohort, fetal schizencephaly was always associated with additional intracranial abnormalities. In most cases, there was evidence that schizencephaly was likely secondary to prior injury. Imaging characteristics may provide clues regarding neurodevelopmental outcome. Postnatal imaging is crucial in assessing the evolution as well as detection of additional abnormalities.
ABBREVIATIONS:
- CC
- corpus callosum
- CM
- cortical malformation
- CP
- cerebral palsy
- DGN
- deep gray nuclei
- GA
- gestational age
- ICH
- intracranial hemorrhage
- IPH
- intraparenchymal hemorrhage
- IQR
- interquartile range
- PMG
- polymicrogyria
- PVNH
- periventricular nodular heterotopia
- SP
- septum pellucidum
- TTTS
- twin-twin transfusion syndrome
- US
- ultrasound
- VM
- ventriculomegaly
SUMMARY
PREVIOUS LITERATURE:
Prior data on the etiology and outcomes of schizencephaly are largely limited to those with postnatal diagnosis. With expanded access to fetal MRI, schizencephaly is increasingly diagnosed in utero, and imaging findings are known to change from fetal to postnatal life. Hence, there is a need to study the association of fetal imaging findings of schizencephaly with etiology and outcomes to guide prenatal work-up and counseling.
KEY FINDINGS:
Fetal schizencephaly was associated with additional intracranial abnormalities, and most (64%) were likely secondary to injury. On postnatal imaging, open defects often involuted, and there were high rates of new findings. Additional cortical malformations were associated with epilepsy, and cerebral palsy and speech delay/intellectual disability were common.
KNOWLEDGE ADVANCEMENT:
Most cases of fetal schizencephaly are likely secondary to injury. Postnatal imaging is necessary to characterize the full spectrum of abnormalities. All children with fetal schizencephaly had neurodevelopmental impairment, though of variable severity, and the presence of additional cortical malformation is associated with epilepsy.
Schizencephaly is a congenital brain anomaly characterized by a gray matter–lined cleft in the cerebral mantle with a prevalence of 1.5 per 100,000 births.1,2 The gray matter–lined lips of the schizencephaly defect may be apposed or not, resulting in closed or open schizencephaly, respectively. The association with non-CNS abnormalities such as gastroschisis, bowel atresia, amniotic band sequence, and facial clefts suggests an acquired vascular insult as the potential etiology.1,3 Schizencephaly is also reported sporadically in association with genetic variants.4⇓⇓–7 However, a definite underlying etiology is often not identified.
In prior literature, fewer than one-half of the patients with schizencephaly were detected prenatally.1,2 With improvement in prenatal testing and the increased availability of fetal MRI, schizencephaly is increasingly diagnosed in utero.2 Nevertheless, reports of prenatally-detected schizencephaly have been limited to small case series.7⇓⇓⇓⇓⇓–13 Furthermore, data on outcomes in schizencephaly have largely been driven by those diagnosed postnatally.14 Prenatally detected schizencephaly may represent a different imaging phenotype compared with those diagnosed postnatally because closed-lip schizencephaly is challenging to diagnose in utero, given the difficulty in resolving the gray matter lining of the cleft on fetal MRI, and the findings of schizencephaly are known to evolve from the fetal to postnatal life.9 Hence, it is difficult to apply the postnatal imaging–based outcomes in the literature to those with prenatally diagnosed schizencephaly, because they may represent different clinical subgroups.
There are limited data available on the association of fetal imaging with etiology and outcomes to guide prenatal work-up and counseling in schizencephaly. We use our large institutional database on fetal MRI spanning >2 decades to comprehensively describe the imaging features, etiology, and outcomes in prenatally diagnosed schizencephaly.
MATERIALS AND METHODS
All fetal MRI reports at our institution (University of California San Francisco) from November 1996 to May 2022 were searched for keywords “schizencephaly” and “schizencephalic.” All identified cases were reviewed by a pediatric neuroradiologist with 5 years’ experience in fetal imaging to identify cases with definitive evidence of fetal schizencephaly and/or postnatal confirmation. Cases with diagnostic uncertainty were adjudicated by consensus review with another pediatric neuroradiologist with 25 years’ experience in fetal imaging. Definitive evidence of fetal schizencephaly included an open defect in the cerebral mantle extending from the ventricle to the subarachnoid space or linear T2 hypointensity suspicious for a closed schizencephaly defect with associated dimpling of the ventricular margin. On postnatal imaging, schizencephaly was defined as a gray matter–lined cleft extending from the ventricular margin to the surface of the brain. The study was approved by the University of California, San Francisco, institutional review board, and the need for informed consent was waived. The manuscript was prepared using the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) reporting guidelines.
Fetal and Postnatal Imaging
The characteristics of the schizencephaly defect were classified, including the number of defects (single versus multiple), location, vascular territory, type (open versus closed), and presence of an overlying membrane. The size of the cleft was assessed by measuring the largest dimension of the open defect in any plane. If multiple fetal MRIs were available, the size was measured on the first MRI. In cases of multiple defects, each was measured separately. Size could not be measured in cases of closed schizencephaly. The presence of other abnormalities was assessed, including intracranial hemorrhage (ICH), ventriculomegaly (VM), cortical malformation (CM), and abnormalities of the deep gray nuclei (DGN), septum pellucidum (SP), corpus callosum (CC), brainstem, and posterior fossa. ICH included germinal matrix hemorrhage, intraventricular hemorrhage, or intraparenchymal hemorrhage (IPH). VM was categorized as mild: 10–11.9 mm, moderate: 12–15 mm, or severe: >15 mm. CM included polymicrogyria (PMG) remote from schizencephaly cleft as well as periventricular nodular heterotopia (PVNH), which was near the schizencephaly defect, remote from the defect, or diffuse (throughout the lateral ventricular margin).
Follow-up fetal MRI and postnatal MRI, when available, were assessed for the evolution of schizencephaly (open to closed or change in size), similar/expected evolution of associated abnormalities, and new abnormalities.
Etiology
Demographics and prenatal and postnatal clinical data were extracted from the electronic medical record. Etiology and clinical outcomes were classified by 2 pediatric neurologists. The cause of schizencephaly was categorized as secondary to prior injury when there was IPH proximate to the cleft, evidence of congenital infection, monochorionic twin gestation with or without twin-twin transfusion syndrome (TTTS), or a maternal/placental risk factor predisposing to ischemia. The cause was classified as potentially genetic when there was evidence of a genetic variant. The etiology was considered unknown when no explanation for schizencephaly was identified despite testing or if no testing information was available.
Clinical Outcomes
Pregnancy and neonatal outcomes included termination of pregnancy, live birth, gestational age (GA) at birth, and survival to >28 days of age. Neurologic outcomes included epilepsy, cerebral palsy (CP), speech delay, intellectual disability, hydrocephalus requiring CSF diversion, and gastrostomy tube dependence.
Statistical Analysis
Descriptive statistics were used, and an association of imaging findings and outcomes was evaluated using the χ2 test, Fisher exact test, and Wilcoxon rank-sum test as appropriate. For fetuses with multiple open defects, the size of the largest defect was used for per-patient analysis. All analyses used Stata 14.0 (StataCorp).
RESULTS
The initial MRI report review identified 37 cases, of which 15 were excluded due to lack of imaging confirmation of schizencephaly (Supplemental Data). Of the remaining 22 fetuses (12 males, 7 females, 3 with unknown sex), 5/22 (22.7%) were twin gestations (3/5 monochorionic diamniotic and 2/5 dichorionic diamniotic). The median maternal age at conception was 31.5 years (interquartile range [IQR], 25.3–35 years), and the median GA at MRI was 22.6 weeks (IQR, 21.9–24 weeks). The earliest GA at which schizencephaly was detected on MRI was 21 weeks. There was evidence of TTTS in 2/5 twin gestations (both former recipient survivors of co-twin demise after laser ablation), and MRI was performed 3–4 weeks after co-twin demise.
Fetal Ultrasound Findings
Fetal ultrasound (US) performed prior to MRI was available in 21/22. The US was performed at a median GA of 20.4 weeks (IQR, 19.4–21.9 weeks) with the median time between US and MRI of 19 days (IQR, 10–23 days). The most common abnormality on US was VM (12/21); mild in 5/11 and bilateral in 7/11 (unknown side and severity in 1 case). Other abnormalities included an absent cavum SP or suspected abnormalities of the CC in 5/21 and an intracranial cyst in 4/21 fetuses. Schizencephaly was suspected on US in 3/21 fetuses. Extracranial abnormalities were diagnosed in 5/21, including multisystem abnormalities (2/5), limb anomalies (2/5), gastroschisis (1/5), and micrognathia (1/5). US findings were normal in 1/21 (performed 12 days after co-twin demise post-laser ablation for TTTS).
Fetal MRI Findings
A total of 34 schizencephaly defects were identified in 22 patients (1–4 per patient). Details of imaging findings are given in Table 1. On per-lesion analysis, the defects were equally distributed between hemispheres (17/34 each) and were open in 28/34 and closed in 6/34. The schizencephaly defects were most commonly frontal (16/34) and in the MCA territory (23/34; 11/23 left and 12/23 right).
Imaging findings in fetal schizencephaly
VM was present in 17/22 and was unilateral in 9/17. All cases had additional intracranial abnormalities (excluding VM) on MRI. There was ICH in 11/22, which was intraparenchymal in 9/22 (all involving the schizencephaly defect). Other abnormalities included an abnormal CC in 10/20 and an abnormal SP (partial or completely absent septal leaflets) in 7/20 (both could not be assessed in 2 fetuses). Other CMs were seen in 13/22, including PVNH and PMG each observed in 9 cases. PVNH was diffuse in 7/9, only at the schizencephaly defect in 1/9, and both at and remote to the defect in 1/9. The PMG was diffuse in 3/9, bilateral in 3/9 (all with unilateral schizencephaly), contralateral to schizencephaly in 2/9, and ipsilateral to schizencephaly in 1/9. The DGN were abnormal in 8/21 (could not be assessed in 1 fetus). The posterior fossa was abnormal in 12/22. The location or number of schizencephaly defects and additional brain abnormalities (except an abnormal CC) were not significantly different among those with and without an abnormal SP. The placenta was abnormal on MRI with heterogeneous T2 signal in 4/20 (could not be assessed in 2 fetuses.).
Follow-up fetal MRI was performed in 2/22 cases at 23–33 weeks GA with the time between MRIs being 10–70 days. The defect was unchanged in size in both (open and closed in 1 each). There were no new abnormalities noted on follow-up fetal MRI; 1 fetus demonstrated expected evolution of IPH and Wallerian degeneration of the ipsilateral cerebral peduncle.
Etiology
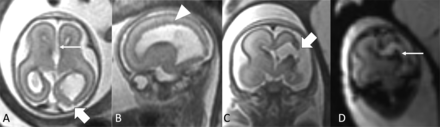
Testing for the etiology was not performed or details of testing were not available in 4/22 fetuses (3/4 underwent termination of pregnancy). Genetic testing was performed in 14/18 fetuses, with microarray in 12/18 and whole exome sequencing in 4/18. Schizencephaly was classified as secondary in 14/22, including 3 monochorionic twins (2/3 with TTTS), 6 with IPH without identifiable cause, 2 with cytomegalovirus infection, and 3 with maternal/placental risk factors (1 mother with homozygous MTHFR variant/systemic hypercoagulability and 2 with fetal thrombotic vasculopathy on placental pathology). Genetic variants were identified in 2 fetuses (one with Bohring-Opitz syndrome and one with variants of uncertain significance in PCDHGA10 and RFX4, Fig 1). The cause of schizencephaly was unknown in 6/22, of whom 5 underwent testing (Table 2).
A 22-week fetus (A and B) with left occipital schizencephaly (block arrow), diffuse bilateral PVNH (arrowhead), callosal agenesis (arrow), and an abnormal brainstem (not shown) with genetic variants in PCDHGA10 and RFX4. A 22-week fetus (C and D) status post laser ablation for TTTS complicated by co-twin demise with left hemispheric encephalomalacia and schizencephaly (block arrow) related to IPH extending to the deep gray nuclei on T2* echo-planar imaging (arrow); secondary etiology.
Imaging findings among those with secondary versus genetic/unknown cause of schizencephaly
Outcomes
Of the 22 fetuses, 8 were liveborn (median GA, 37.8 weeks; IQR, 35.6–39.5 weeks), one was stillborn, and 13 underwent termination. Of the 8 liveborn, one died on day 1 of life. The last known follow-up was at a median of 4 years (range, 7 months to 21 years). Of those who survived beyond the neonatal period, 1/7 had shunted hydrocephalus, 4/7 had epilepsy, 1/7 was gastrostomy tube–dependent. Neurodevelopmental outcomes were available in 5/7; 4/5 had CP, 3/5 had speech delay, and 1/5 had intellectual disability. CP was present in all cases with an open defect (open: 4/4 versus closed 0/1, P = .20). The median size of the defect was larger in the patient with severe motor impairment compared with those with mild-to-moderate motor impairment (41 versus 12 mm, P = .18). All those with epilepsy had CM remote from the schizencephaly (4/4 versus 0/3 without epilepsy, P = .03, Fig 2). Only 1/4 with severe fetal VM required postnatal shunting.
A 32-week fetus with monochorionic diamniotic gestation with 1 twin demonstrating large temporoparietal schizencephaly (block arrow), extensive cortical malformation remote from the defect (arrows), and large posterior fossa cyst causing mass effect on brainstem and cerebellum (arrowhead). No etiology was identified. The patient is 21 years of age at follow-up with severe motor impairment, nonverbal intellectual disability, epilepsy, shunted hydrocephalus, and gastrostomy tube dependence.
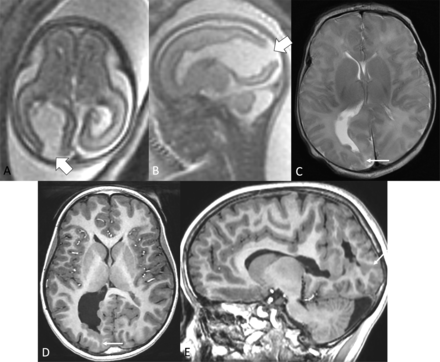
Postnatal imaging was available in 6/8, performed at a median of 6.5 days of life (range, 1–480 days). Of the open defects on fetal MRI, 7/11 became closed on postnatal MRI (Fig 3), 1/11 was smaller in size, and 3/11 were similar in size. Only 3/6 had blood-sensitive postnatal imaging sequences, and none of these had evidence of blood products along the schizencephaly defect (one had evidence of IPH on fetal MRI.). There was evidence of an overlying membrane on postnatal imaging in 2/6, both of which were present on fetal MRI (Fig 4). The other 4/6 had no overlying membrane on fetal or postnatal imaging. Additional findings identified only on postnatal imaging included germinal matrix hemorrhage (1/6 in a preterm infant), abnormal DGN (2/6), periventricular calcifications (1/6 in subject with cytomegalovirus infection), a thin CC (1/6), and bilateral optic nerve hypoplasia (1/6). GA at fetal MRI, the size of the schizencephaly defect, and the presence of an overlying membrane were not associated with postnatal closure of the defect (P = .92, P = .21, and P = .36, respectively).
A 21-week fetus with right occipital open-lip schizencephaly (block arrow, A and B), which involuted to closed-lip schizencephaly (arrow, C) on postnatal MRI at 2 days of life and is also seen at 8 years of life (arrow, D and E).
A 32-week fetus with right temporal open-lip schizencephaly (block arrow, A) with an overlying membrane (arrow, A), which persisted at 1 year of life as an open defect (block arrow, B) with overlying membrane (arrow, B).
DISCUSSION
There are limited data on the imaging findings, etiology, and developmental outcomes in fetal schizencephaly, posing a challenge for evidence-based prenatal work-up and counseling. In this largest cohort to date of prenatally detected schizencephaly, we identified evidence of injury in 64%, including vascular, infectious, and placental causes, and 2 cases with a potential genetic cause. Among those with follow-up, all children had neurodevelopmental impairment of variable severity, and the presence of additional CMs was associated with epilepsy.
In our cohort, most schizencephaly defects (73%) were open, similar to those in prior fetal reports (55%–100%)8⇓–10 but slightly higher than the wide range reported in children (24%–85%).3,10,14,15 This feature may be due to the challenges in diagnosing closed schizencephaly in utero, as well as the evolution of the defects from open to closed during fetal-to-postnatal life.9⇓–11 We demonstrated that 73% had a decrease in the size of schizencephaly defects from fetal to postnatal life, similar to prior reports (47%–75%).9,11 In our cohort, there was evidence of a membrane overlying the schizencephaly defect in 47% (16/34), which is similar to a prior report of 55% (10/18).9 On postnatal imaging, a fluid collection overlying the cleft was reported in 33%, with an overlying membrane visible in only 2%–11%.9,16 Prior literature suggested that the overlying membrane seen on fetal MRI may not be identified on postnatal imaging, potentially due to defect closure or membrane rupture,9 though this was not represented in our cohort.
Multiple schizencephaly defects occurred in 36%, which is within the wide range that has been reported (35%–80%).3,9,10,14 While frontal lobe location was most common, the defects were more evenly distributed among various lobes in our cohort compared with prior reports of 60%–65% frontal and parietal defects.3 Additional findings in this cohort are slightly different from prior smaller reports, likely due to the heterogeneity within this rare anomaly and the larger size of our cohort. Septo-optic dysplasia is associated with schizencephaly, with septal abnormalities reported in 64%–90% of fetuses and 70%–76% of children, slightly higher than that in our cohort (35%).3,9,10 Prior studies have suggested an association of septal defects with open10 and frontal defects,17 which we did not observe. Our cohort had a higher prevalence of associated CM (59%) than previously reported in fetuses (20%-45%),10 but similar to that reported in children (57%–66%).10
Fetal MRI provided additional information in all cases, and schizencephaly was suspected on US in only 3/24 (12.5%). In a prior registry-based study, 33% of antenatally detected schizencephaly cases were identified on US, though none of these were closed-lip.2 This difference may be due to the relatively high rate of closed-lip schizencephaly in our cohort (27%), lack of a dedicated neurosonogram, and easy access to fetal MRI in our practice. Additional prior reports include small case series limiting direct comparison of MRI and US-based detection rates.11 Furthermore, fetal MRI characterized additional CNS abnormalities relevant for prognostication. Postnatal imaging revealed multiple additional findings, underscoring its utility, similar to those in a prior report.9
The yield of diagnostic testing was 45% (10/22, excluding IPH without identifiable cause), which is higher than previously reported (20%).11 In a majority, the cause was likely secondary to injury, such as from IPH, monochorionic twin gestation, infection, or maternal/placental risk factor. Additional reported etiologies not represented in our cohort included alcohol or drug use, maternal trauma, and alloimmune thrombocytopenia.18⇓⇓–21 Young maternal age has been reported as a risk factor for schizencephaly,1,2,22 though in our cohort, the median maternal age was 31.5 years, which may be related to regional differences in demographics. Interestingly, schizencephaly defects in our cohort were predominantly in the MCA territory, similar to the known distribution of perinatal infarcts23 but were equally distributed between the left and right hemispheres, unlike the left-sided predominance of perinatal arterial ischemic infarcts.23 The high prevalence of IPH (41%) in our cohort also supports a vascular injury mechanism. In the literature, genetic causes are rarely identified, and candidate genes such as EMX2 or SIX have been proposed.4,5 Bohring-Opitz syndrome due to the pathogenic variant in ASXL1 was identified in 1 subject in our cohort. This is associated with callosal abnormalities, Dandy-Walker malformation, VM, and PVNH, but, to our knowledge, schizencephaly has not been reported.24 Genetic causes for vascular injury and secondary schizencephaly25 include COL4A1/2-related disorders, which are not represented in our cohort.26 The yield of genetic testing in schizencephaly is unclear but warrants further investigation.
Determination of the neurodevelopmental outcome after fetal diagnosis of schizencephaly is limited due to censoring bias from the high rate of termination. Of the small number with clinical follow-up, CP, speech delay, and epilepsy were observed frequently, though not universally. The single prior report of outcomes in fetal schizencephaly found that 75% (3/4) had moderate-to-severe neurodevelopmental impairment.11 Prior studies of patients with postnatally diagnosed schizencephaly (2–20 patients) suggest that large and bilateral clefts are associated with worse motor, speech, and intellectual outcomes3,14,15,27⇓–29 and frontal lobe involvement is associated with worse motor outcomes.14 In our cohort, among those older than 1 year of age, all 4 with open fetal defects had CP compared with 0/1 with a closed defect, though this finding was not statistically significant.
Our cohort also demonstrates that cases with epilepsy had CM remote from the schizencephaly defect. The extent of brain injury in schizencephaly is not associated with epilepsy in postnatal studies.29 Remote CM has not been comprehensively assessed previously and represents an important observation from our cohort. A prior study has suggested worse neurodevelopmental outcomes and medically refractory epilepsy among neonates with schizencephaly and septo-optic dysplasia.30 There was 1 patient with optic nerve hypoplasia diagnosed postnatally, but none of our patients had endocrine abnormalities. The relationship between imaging findings and neurodevelopmental outcome in schizencephaly is likely mediated by the timing of the insult and the diagnosis (fetal versus postnatal), as well as the underlying etiology.
Our retrospective study has several limitations. Details about etiology and neurologic outcomes were determined by data extraction from clinical charts, which has inherent limitations due to variable clinical practice. Both fetal imaging techniques and prenatal diagnostic work-up including access to genetic testing have changed during the study duration. Thus, identification of etiology (particularly genetic causes) may be underreported in this cohort. Finally, the high rates of termination limit our postnatal neurologic outcome assessment, but are reflective of clinical practice.12
CONCLUSIONS
Fetal schizencephaly is a rare brain anomaly, which is associated with additional intracranial abnormalities and most are likely secondary to in utero injury. Fetal schizencephaly is associated with high rates of cerebral palsy and epilepsy, and imaging findings may be associated with the neurodevelopmental outcome. Further research on outcomes in larger multicenter cohorts is necessary to inform accurate prognostication in pregnancies of fetuses with schizencephaly.
Footnotes
This work was supported by the American Society of Neuroradiology, Scholar grant 2020, and the Radiological Society of North America, Scholar grant 2023.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received June 26, 2024.
- Accepted after revision October 2, 2024.
- © 2025 by American Journal of Neuroradiology