SUMMARY:
Dextromethorphan toxicity in young children (especially those 4 years of age or younger) can have an extremely poor prognosis if untreated. However, if timely recognized and optimally managed, it can have a good clinical outcome despite a profound initial insult. We present 3 pediatric cases (younger than 5 years of age) with sudden unresponsiveness following ingestion of cough medications containing dextromethorphan. All these children showed cytotoxic edema in the cerebellar hemispheres on MR of the brain, with diffusion-restricting foci in the supratentorial white matter in 2 patients. These features resemble the recently described acute opioid toxidrome in children, pediatric opioid use–associated neurotoxicity with cerebellar edema (POUNCE). Hence, we named this entity dextromethorphan-associated neurotoxicity with cerebellar edema (DANCE) to increase the awareness of dextromethorphan toxicity in young children and the need to promptly recognize it to initiate optimal management.
ABBREVIATIONS:
- DANCE
- dextromethorphan-associated neurotoxicity with cerebellar edema
- IgG
- immunoglobulin G
- POUNCE
- pediatric opioid use–associated neurotoxicity with cerebellar edema
Dextromethorphan-associated neurotoxicity in young children is less well-described in the literature. POUNCE syndrome, the term used for pediatric opioid use–associated neurotoxicity with cerebellar edema is a recently recognized clinico-radiologic entity in children, occurring secondary to acute opioid toxicity. It is extremely rare with fewer than 20 cases published in the literature.1 We report 3 pediatric patients (younger than 5 years of age) who presented to our institution during a span of 2 years with clinical and radiologic features like POUNCE syndrome but with an antecedent history of ingestion of over-the-counter cough medications containing dextromethorphan for fever, cough, and coryza. We, therefore, named this entity dextromethorphan-associated neurotoxicity with cerebellar edema (DANCE) syndrome due to its striking resemblance to the POUNCE syndrome. We consider it different from POUNCE syndrome because it did not occur directly due to opioid ingestion, rather occurred secondary to ingestion of an opioid analog with a slightly different mechanism of action. Our intention is to increase awareness of this entity in children 4 years of age or younger for whom over-the-counter antitussive medications containing dextromethorphan are otherwise not recommended but are still used in a few parts of the world.
CASE SERIES
Clinical Presentation
Case 1.
A 4-year-old girl presented with low-grade fever, cough, and coryza for 2 days. She was given a cough syrup containing dextromethorphan hydrobromide (dextromethorphan dose of 1 mg/kg), chlorpheniramine maleate, and phenylephrine hydrochloride from a local physician. Three hours after ingestion of the cough syrup, she presented with sudden unresponsiveness. A similar presentation was elicited at the same time in her younger sibling of 2 years of age, who died before reaching hospital. There was no history of any animal bites or exposure to other toxins like organophosphorus compounds or opioids. At arrival, the child was unconscious with a Glasgow Coma Scale score of 4 (E1V1M2) and had bilateral pinpoint pupils. She was febrile and tachycardic and had an elevated blood pressure of 131/67 mm Hg, a low oxygen saturation level of 75% at room air, and a low blood glucose level of 43 mg/L. Examination revealed generalized hypotonia with brisk deep tendon reflexes and bilateral extensor plantar.
Case 2.
A 2-year-old boy presented with fever and upper respiratory tract illness for 1 day, for which a cough syrup containing dextromethorphan hydrobromide (dextromethorphan dose of 1.6 mg/kg) was prescribed in a local clinic. One hour after ingestion of the syrup, the child was found to be unresponsive. On the way to the hospital, he had 7 brief episodes of generalized tonic-clonic seizures, each lasting for 3–5 minutes. On arrival, the child was unconscious and febrile and had tachycardia, bilateral pinpoint pupils, and generalized hypotonia with brisk deep tendon reflexes and bilateral extensor plantar.
Case 3.
A 1.5-year-old girl presented with intermittent fever and dry cough for 2–3 days and decreased responsiveness for the last 2 hours. She had an episode of abnormal body movements in the form of clenching of teeth and frothing from mouth, with bluish discoloration of the body 1 hour before reaching our hospital. She had been given a similar oral cough syrup containing dextromethorphan hydrobromide like the previous 2 patients at a local private hospital for her respiratory illness. On examination, the child was lethargic with Glasgow Coma Scale of 7 (E1V2M4) and had bilateral constricted pupils. She developed respiratory failure with a venous oxygen saturation level of 54% at room air and was found to have hypoglycemia.
All the children had normal CSF examination findings. Patient 1 had positive immunoglobulin G (IgG) antibodies for coronavirus disease 2019 (COVID-19). Hematologic and biochemical investigations revealed elevated transaminases (AST/ALT [IU/L] in patient 1: 56/197, patient 2: 118/41, and patient 3: 128.9/43.9). Patient 3 had raised creatine kinase–MB level (61.4) and creatine kinase level (1095) with hyperkalemia, indicating rhabdomyolysis. She also had a high interleukin 6 value of 133 pg/mL (normal value: <4.4).
Imaging Findings
After initial resuscitation, the patients underwent MRI of the brain within 6 hours of their presentation. MRI brain in all patients consistently showed confluent T2-FLAIR hyperintensities in the bilateral cerebellar hemispheres with restricted diffusion on the corresponding DWI sequence, suggestive of cytotoxic edema. Multiple other punctate and linear foci of restricted diffusion were seen in the bilateral centrum semiovale and corona radiata in patients 1 and 2 (Figs 1 and 2). Patient 3 did not have supratentorial involvement. The basal ganglia or hippocampi were not involved except in patient 2 in whom a few diffusion-restricting foci were seen in the left caudate nucleus and bilateral posterior putamina (Fig 2). None of the patients showed cerebral cortical involvement, blooming/bleeds on SWI, enhancement on postcontrast T1-weighted sequences, or any evidence of vascular stenosis/occlusion on TOF-MRA. These features strongly resembled the recently described typical features of POUNCE syndrome.1
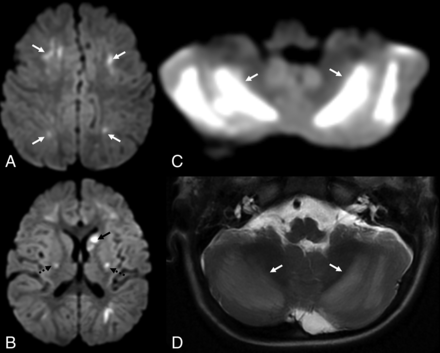
Image of a 4-year-old girl who presented with sudden unresponsiveness and pinpoint pupils 3 hours following ingestion of a cough syrup containing dextromethorphan hydrobromide (dextromethorphan dose of 1 mg/kg), prescribed to her for low-grade fever, cough, and coryza from an outside hospital. Axial MR brain images: diffusion-weighted images (A and C) and their corresponding ADC maps (B and D) show near-symmetric linear diffusion-restricting foci in the bilateral centrum semiovale (arrows in A and B). Symmetric and confluent diffusion-restricting areas are seen in the bilateral cerebellar hemispheres (arrows in C and D), indicating cerebellar edema.
Image of a 2-year-old boy who presented with unresponsiveness, brief episodes of generalized tonic-clonic seizures, and bilateral pinpoint pupils 1 hour following ingestion of a cough syrup containing dextromethorphan hydrobromide (dextromethorphan dose of 1.6 mg/kg), prescribed to him for upper respiratory tract illness. Axial MR brain images (A, B, and C, Diffusion-weighted sequence. D, T2-weighted sequence) show features of DANCE syndrome. Symmetric linear diffusion-restricting foci with corresponding low values on the ADC map (not shown) are seen in the bilateral centrum semiovale (arrows in A) and deep white matter (B). A few diffusion-restricting foci are seen in the left caudate nucleus (black solid arrow in B) and bilateral posterior putamina (black dashed arrows in B). Confluent areas of diffusion restriction (with low values on the ADC map, not shown) are seen in the bilateral cerebellar hemispheres (arrows in C), with corresponding hyperintense signal on T2-weighted images (arrows in D).
Treatment and Follow-Up
Emergent measures were performed in all the children by securing the airway with intubation and administering a 10% dextrose bolus for hypoglycemia. Considering dextromethorphan-induced encephalopathy, they were given bolus doses of naloxone followed by an infusion for 48 hours along with measures to reduce intracranial pressure. In view of cerebellar edema, they were given methylprednisolone pulse therapy for 5 days, followed by oral prednisolone for 2 weeks. All the children showed dramatic improvement in the sensorium after methylprednisolone therapy. They were discharged in stable condition without any residual deficits after a brief hospital stay varying from 5 to 10 days and had normal neurologic examination findings on a 2-week follow-up. Follow-up MR 2 weeks later in patient 3 showed significant resolution of T2-FLAIR hyperintensities in the cerebellum with normalization of restricted diffusion (Fig 3). The Online Supplemental Data summarize the clinical and imaging features, treatment, and follow-up of the patients in the clinical report.
Image of a 1.5-year-old girl who ingested oral cough syrup containing dextromethorphan hydrobromide for intermittent fever and dry cough and now presented with decreased responsiveness, a seizure episode, and bilateral constricted pupils. Axial MR brain images (A, Diffusion- weighted sequence. B, ADC map. C, T2-weighted sequence) show features of DANCE syndrome. Symmetric and confluent areas of restricted diffusion (arrows in A) with low values on the ADC map (arrows in B) and corresponding hyperintense signal on T2-weighted sequence (arrows in C) are seen in the bilateral cerebellar hemispheres, indicating cerebellar edema. No diffusion-restricting foci were seen in supratentorial white matter in this case (not shown). D, Follow-up MR image (diffusion-weighted sequence) obtained 2 weeks later in the patient shows no residual diffusion restriction in cerebellum.
DISCUSSION
Dextromethorphan-associated neurotoxicity in young children is a little-known entity. We describe 3 pediatric patients who presented with significant neurologic deterioration after ingestion of over-the-counter antitussive medications containing dextromethorphan for their short febrile respiratory illness. The patients in our series had strikingly similar clinical features and MRI of the brain appearances like those of the recently described POUNCE syndrome.1
POUNCE syndrome is a term coined by Kim et al1 for a distinct clinico-radiologic syndrome caused by acute opioid toxicity in children. It is characterized by cerebellar edema in the form of T2-FLAIR hyperintensities with or without restricted diffusion. It is often accompanied by some degree of supratentorial white matter injury, predominantly in the deep and periventricular white matter, seen as punctate or confluent T2-FLAIR hyperintensities, which may show restricted diffusion in the early stages.1 A few of the previously published reports on pediatric opioid toxicity have also described similar cerebellar and white matter injury, often with malignant cerebellar edema leading to complications, including cerebellar tonsillar herniation, compression of brainstem, and rapidly evolving obstructive hydrocephalus. These complications may lead to dreaded consequences, including death if not addressed in early stages by antiedema measures or posterior fossa decompression or placement of an external ventricular drainage tube if necessary.1⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓-12
All the children in our report presented with profound encephalopathy and respiratory failure and had cerebellar edema on MRI. Two of them showed supratentorial involvement. None of the children showed hemorrhages or contrast enhancement, though hemorrhage has been reported in a few of the previously published cases of POUNCE.6 Our patients did not show significant compression of the brainstem or upstream hydrocephalus and did not require surgical measures, likely due to early recognition and administration of appropriate therapy.
Dextromethorphan, an over-the-counter antitussive medication, is a D isomer of levorphanol, which is a synthetic analog of codeine, an opioid receptor agonist. Its cough-suppressant actions in regular doses are mediated via its action on σ opioid receptors in the medulla. Due to its stereochemistry, it does not bind to μ and δ opioid receptors, which are commonly involved in manifestations of opioid toxicity.13,14
Although definite histopathologic evidence is lacking to date, POUNCE is postulated to occur due to direct neurotoxic effects of the opioid agents on glial and neuronal cells, with contributions from factors like apoptotic upregulation due to mitochondrial injury and potentiation of these mitochondrial pathways from anoxic insults secondary to respiratory depression.1,2,4 Predominant cerebellar involvement in POUNCE is proposed due to an abundance of μ receptors in the cerebellum, which are the primary target for opioid toxicity.1
Although dextromethorphan primarily binds with σ opioid receptors, due to a striking resemblance of the clinical and radiologic syndrome in all the cases of our series with POUNCE, we postulate the occurrence of DANCE syndrome in small children with dextromethorphan toxicity and propose a similar mechanism with the possibility of cross-reactivity with μ receptors. Over-the-counter cough remedies containing dextromethorphan have been largely prohibited for children younger than 2 years of age and are not recommended for use in those younger than 4 years of age.14⇓-16 The occurrence of DANCE syndrome in young children in our report further emphasizes the necessity of this precaution. A recently published case report has highlighted clinical features of opioid toxidrome secondary to dextromethorphan toxicity in children, but it is extremely rare and there is a paucity of knowledge about the associated neuroimaging features.17 Although never proved, the occurrence of dextromethorphan toxicity in only young children may be due to the enhanced sensitivity of the receptors in this age group, leading to a heightened effect even at doses less than the toxic range. Further research into this matter is warranted.
Because all the children in our series had a respiratory illness and showed good response to methylprednisolone, one of them had high interleukin-6 levels, and one had positive IgG for COVID-19, the possibility of an underlying parainfectious immunologic phenomenon and the role of viral infection in the increasing predisposition to DANCE syndrome also need to be investigated. In this context, the most important differential of DANCE syndrome remains viral/infectious cerebellitis. However, a specific MR pattern involving both the cerebellum (in all the children) and supratentorial white matter (in 2 of the 3), a remarkably similar clinical context with features including pinpoint pupils, a striking resemblance to POUNCE syndrome, a lack of contrast enhancement, and normal findings on blood and CSF investigations despite extensive infectious disease work-up make the diagnosis of an infectious/viral cerebellitis less likely than DANCE. Hypoxic-ischemic encephalopathy is another major clinical differential of DANCE syndrome in patients with sudden unresponsiveness and respiratory failure. Predominant involvement of the cerebellar white matter and centrum semiovale without cortical and basal ganglia involvement on MRI of the brain makes the distinction from hypoxic-ischemic encephalopathy possible, when the cerebellum is usually involved late.1,18,19
A similar predilection for the cerebellum and white matter in cases of “chasing the dragon” leukoencephalopathy occurring due to chronic rather than acute inhalation of heroin and the recently described cerebellar, hippocampal, and basal nuclei transient edema with restricted diffusion syndrome and opioid-associated amnestic syndrome occurring secondary to acute opioid toxicity in adults suggests that all these disorders may represent a continuum of similar pathophysiology.18⇓⇓-21 Nevertheless, there are differences (Online Supplemental Data) among these entities possibly due to varying binding affinity of opioid receptors in different parts of the brain in children and adults.22
Our report has a few limitations. We could not determine the serum levels of dextromethorphan in the children in our series. The lack of supratentorial involvement in the third child in our study could not be explained. This may be related to the difference in the sensitivity of the brain receptors at different ages because the third child was the youngest of the 3 (younger than 2 years of age), but we refrain from making a definite assumption without any histopathologic or other conclusive evidence from the literature. Due to logistic reasons, MR follow-up was not available in 2 of the 3 patients. However, normal neurologic examination findings were reassuring and indicated a definite clinical improvement in these patients.
DANCE syndrome can have an extremely poor prognosis if untreated as could be seen in the sibling of the patient in case 1 who succumbed to death before reaching our hospital. However, if timely recognized and optimally managed, it can have a good clinical outcome despite a significant initial insult as seen in all the patients of our series, likely owing to the neuroplasticity of the pediatric brain.1 This experience highlights the importance of early neuroimaging and the need to promptly recognize the specific DANCE pattern and urgently treat this rare entity. The case series reiterates the adverse effects of dextromethorphan in young children and the need to monitor its use, especially in children younger than 4 years of age.
Acknowledgments
We sincerely thank Dr Venkata Subbaih Arunachalam, DM, resident in Cardiovascular Imaging and Vascular Interventional Radiology for help in drawing illustrations represented in the Online Supplemental Data of the article.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received June 8, 2024.
- Accepted after revision August 7, 2024.
- © 2025 by American Journal of Neuroradiology