SUMMARY:
Glucose transporter type 1 deficiency syndrome (GLUT1-DS) is an uncommon condition represented by an infantile-onset disorder, frequently arising from heterozygous mutations in the SLC2A1 gene. Individuals with GLUT1-DS may present with early-onset seizures (typically manifesting before 4 years of age), developmental delay, and complex movement disorders. In fewer cases, stroke-like events or hemiplegic migraine-like symptoms are also reported, defined by unilateral paresis affecting 1 side of the body and/or one-half of the face, occasionally accompanied by speech impairment. Currently, the pathomechanism underlying these acute transient clinical manifestations is poorly understood. MR imaging studies performed in the absence of acute manifestations frequently reveal nonspecific imaging signs associated with this syndrome. We present findings obtained using the arterial spin-labeling technique for perfusion imaging and MRA during the acute onset of stroke-like episodes in a series of 4 pediatric patients with GLUT1-DS. We observed reversible hypoperfusion in the left hemisphere and associated reversible attenuation of distal MCA branches on MRA. A notable association between unilateral cerebral hypoperfusion and transient crossed cerebellar diaschisis was evident on perfusion maps as well.
ABBREVIATIONS:
- ASL
- arterial spin-labeling
- EEG
- electroencephalography
- GLUT1
- glucose transporter 1
- GLUT1-DS
- glucose transporter type 1 deficiency syndrome
- pCASL
- pseudocontinuous arterial spin-labeling
Glucose transporter type 1 deficiency syndrome (GLUT1-DS) is a rare congenital disease caused by a deficiency of glucose transporter 1 (GLUT1), which is primarily expressed in endothelial cells and astrocytes forming the BBB. The main gene involved is SLC2A1, with most cases resulting from autosomal dominant de novo heterozygous mutations.1 The hallmark of the disease is reduced glucose concentration in the CSF, despite normoglycemia.
Epilepsy is frequently the initial symptom, with absence seizures (before 4 years of age) being the most common presentation; generalized seizures are more likely to occur than focal ones.1⇓-3 The most frequent electroencephalographic (EEG) abnormalities are 2.5- to 4-Hz generalized spike and wave discharges,4,5 though interictal EEG often has normal findings.6 Within the spectrum of GLUT1-DS, spasticity, ataxia, and movement disorders such as dystonia, chorea, and tremor have also been described.1,3 Microcephaly and intellectual disability, ranging from mild to severe, may also be present.1 It has been recently reported that GLUT1-DS-affected patients may experience recurrent acute transient focal neurologic deficits, including episodes that could be classified as alternating hemiplegia of childhood, hemiplegic migraine, or more generally stroke-like events.1,7 The exact percentage of patients with GLUT1-DS who develop stroke-like events is not known, though in a study of 42 patients between 6 and 38 years of age, 8 presented with an acute onset of neurologic disturbances.8
Despite the growing awareness of the phenotype associated with the disorder, there is a limited understanding of the underlying mechanisms by which low GLUT1 contributes to brain dysfunction. The energy deficit related to this uniport channel impairment seems to play the main role in GLUT1-DS, and a ketogenic diet, as an alternative energy source to brain metabolic needs, is reported to control epilepsy and motor symptoms.9 GLUT1 is also involved in endothelial metabolism and function, with implications for CNS development and BBB integrity.10
Patients with GLUT1-DS have been reported to have nonspecific subcortical white matter T2-weighted hyperintensities, prominent Virchow-Robin spaces, and delayed myelination; however, approximately 25% present with normal findings on brain MR imaging.1 Possible imaging findings related to stroke-like events have been poorly investigated. In this report, we present novel MR imaging findings from a series of 4 patients with GLUT1-DS. These patients, despite ketogenic therapy, experienced stroke-like events, and they underwent imaging during the acute phase. All patients were diagnosed with pathogenic variants in the SLC2A1 gene and underwent a lumbar puncture to test glucose levels in the CSF; all of them had a glycemia/glycorrhachia ratio lower than 0.5 (see the Online Supplemental Data for details). Genetic testing excluded mutations in genes related to hemiplegic migraine (CACNA1A) and alternating hemiplegia of childhood (ATP1A2).
CASE SERIES
This was a single-center retrospective study, reporting 4 patients.
These cases were collected as consecutive imaging observations during the acute onset of stroke-like episodes and were followed up between the third and seventh day. Patients’ imaging data were retrieved and evaluated from the PACS and internal medical records between 2022 and 2024. Imaging assessment was performed by our senior author (A.R. with >20 years’ experience in pediatric neuroradiology). The study complied with institutional regulations for anonymized retrospective studies and was related to the approved study by the ethics committee of Area 1 of Milan (2021/ST/004).
MR procedures were performed on the latest-generation 3T scanner (Magnetom Vida; Siemens) for case 1, case 2, and case 4, and on a 1.5 T scanner (Ingenia; Philips Healthcare) for case 3, including conventional T1- and T2-weighted sequences, 2D T2 FLAIR, arterial spin-labeling (ASL) by a 3D pulsed continuous arterial spin-labeling (pCASL) acquisition, DWI, and TOF-MRA. In cases 2 and 3, SWI was also performed, and in case 2, single-voxel (TE = 144 ms) proton MRS was performed during the acute phase .
For details about ASL sequence parameters and data analysis refer to the Online Supplemental Data.
Case 1
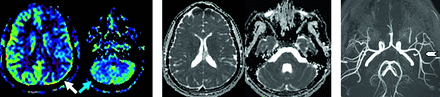
A 12-year-old girl presented with acute-onset aphasia, ataxia, dysmetria, vomiting, and right-arm weakness. Ketone levels at onset were 0.6 mmol/L, increasing to 2.9 mmol/L within 4 hours. EEG showed a slow activity in the left hemisphere with slower δ-θ waves in the anterior and left temporal regions (Online Supplemental Data). No abnormal findings were detected on T2 and T2 FLAIR sequences, while 3D-pCASL revealed hypoperfusion in the left cerebral hemisphere, accompanied by hypoperfusion in the contralateral cerebellum Fig 1). Additionally, MRA showed a diminished representation of distal branches of the left MCA. Within 24 hours, symptoms totally subsided. A follow-up examination 4 days later showed resolution of all abnormalities. No focal signal anomalies were ever noted on conventional imaging and ADC maps.
Case 1. MR examinations from left to right: 3D-pCASL, ADC map, and MRA-MIP. The upper row shows images acquired during the acute phase: hypoperfusion in the left cerebral hemisphere (white arrow) and in the contralateral cerebellar hemisphere (blue arrow). No ADC map changes are seen. Note reduced representation of the distal branches of the left MCA (white arrowhead). The lower row shows the follow-up examination 4 days later with normalization of all the findings.
Case 2
A 7-year-old girl presented with symptoms including acute-onset aphasia, dysarthria, sialorrhea, confusion, irritability, and right-arm weakness. After 2 hours, she developed headache and vomiting. Initial ketone levels were 2.6 mmol/L. EEG showed interhemispheric asymmetry with hypovolted activity in the left hemisphere, while normal background activity was detected in the right one (Online Supplemental Data). The MR examination did not show any findings on T2 and T2 FLAIR sequences; a perfusion study demonstrated left cerebral hemisphere hypoperfusion on 3D-pCASL and bilateral cerebellar hypoperfusion, more pronounced in the contralateral hemisphere (Fig 2). SWI showed a mild-to-moderate increase in the conspicuity of cortical veins on the left side, and MRA revealed a reduction in the representation of distal branches of the left MCA. Proton MRS showed no lactate peak (Online Supplemental Data). The patient fully recovered within 6 hours. On follow-up 3 days later, all abnormalities, including SWI findings, had resolved on MR imaging. No focal signal anomalies were ever noted on conventional imaging and ADC maps.
Case 2. MR examinations from left to right: 3D-pCASL, ADC map, MRA-MIP, and SWI. The upper row shows the acute onset: hypoperfusion in the left cerebral hemisphere (white arrow) and in cerebellar hemispheres but more pronounced in the right cerebellar hemisphere (blue arrow). No ADC map changes are seen. Note reduced representation of the distal branches of the left MCA (white arrowhead) and a mild increase in the conspicuity of cortical veins on the left side on SWI (blue arrowhead). The lower row shows the follow-up examination after 3 days, with normalization of all the findings.
Case 3
A 16-year-old girl presented with acute onset of right-arm weakness, dysarthria, and a right-sided facial nerve deficit. Within 30 minutes, only aphasia and the facial deficit persisted. Her initial ketone levels were 1.2 mmol/L (Online Supplemental Data). EEG was not performed during the acute phase. The MR scan revealed left cerebral hemispheric hypoperfusion, along with a mild reduction in the contralateral cerebellar hemisphere (Fig 3). Notably, a cortical atrophic-gliotic area was identified in the right cerebellar hemisphere, but no additional findings were detected on T2 and T2 FLAIR sequences. SWI showed a mild increase in the conspicuity of cortical veins on the left side, and MRA indicated a reduction in the representation of distal branches of the left MCA. A follow-up 7 days later showed normalization of all acute findings, including on SWI. No focal signal anomalies were ever noted on the ADC map.
Case 3. MR examinations from left to right: 3D-pCASL, ADC map, TOF-MIP, and SWI. The upper row shows the acute phase findings: hypoperfusion of the left cerebral hemisphere (white arrow) and of the contralateral cerebellar hemisphere (blue arrow). No ADC map changes are seen. Note reduced representation of distal branches of the left MCA (white arrowhead) and a mild increase in the conspicuity of cortical veins on the left side on SWI (blue arrowhead). The lower row shows the follow-up examination after 7 days, with normalization of all acute findings. There is persistent-but-improved hypoperfusion of the right cerebellar hemisphere proportional to what would be expected for the known background encephalomalacia in this region (blue arrow).
Case 4
A 15-year-old girl presented with acute-onset headache, right-arm weakness, and pain, followed by multiple vomiting episodes within 30 minutes. Her initial ketone levels were 1.5 mmol/L. EEG showed slow activity in the left hemisphere with slow epileptic discharges in the frontal centrotemporal regions (Online Supplemental Data). The MR examination did not show any findings on T2 and T2 FLAIR sequences; the perfusion study revealed hypoperfusion in the left cerebral hemisphere on 3D-pCASL and a mild reduction in perfusion in the contralateral cerebellar hemisphere (Fig 4). Additionally, MRA demonstrated a decreased representation of distal branches of the left MCA. No focal signal anomalies were noted on conventional imaging or ADC maps. No follow-up MR study was performed.
Case 4. MR examination from left to right: 3D-pCASL, ADC map, and MRA at the acute onset of symptoms: hypoperfusion of the left cerebral hemisphere (white arrow) and of the contralateral cerebellar hemisphere (blue arrow). No ADC map changes are seen. Note reduced representation of the distal branches of the left MCA (white arrowhead).
DISCUSSION
All presented patients have common features of acute symptom onset caused by single cerebral hemisphere disfunction, accompanied by a congruous unilateral cerebral hemisphere (the left one) reversible hypoperfusion, reduced MRA arterial representation, and decreased EEG activity (Online Supplemental Data). Notably, a contralateral cerebellar hemisphere transient hypoperfusion was also noted as a novel sign of very likely functional crossed cerebellar diaschisis in these subjects with GLUT1-DS. The predominant symptoms in patients with GLUT1-DS seem to depend on the age of the patient. In younger patients, GLUT1-DS manifests as clusters of tonic-clonic seizures, absence seizures, and myoclonus. Meanwhile, in older patients, it presents as paroxysmal dyskinesia or paroxysmal weakness of the lower limbs. Muscle stiffness, gait abnormalities, and dysarthria are also evident in older patients. Behavioral problems are reported as remarkable features within the spectrum related to this condition. Therefore, GLUT1-DS should be considered a condition with a large spectrum of symptoms with varying degrees of severity. According to the work of Winczewska-Wiktor et al,11 the presentation can vary widely, even within the same family and with the same genetic background. The ketogenic diet has been shown to be very effective in treating epilepsy and, to a lesser extent, motor and behavioral problems. In some cases, such as the one reported by Klepper et al,12 it has also been effective to counter delayed demyelination.
Currently, conventional MR imaging does not have a specific diagnostic role in GLUT1-DS management; rather, it is predominantly performed to rule out alternative etiologies such as stroke in children, because conventional imaging findings are frequently normal13⇓-15 or may show some delayed myelination.2,15 Additionally, we were able to evaluate the presence of crossed cerebellar diaschisis, which had not been previously reported. Our case series differs from others because it includes only pediatric patients for whom a complete follow-up protocol with MRI and pCASL was available. Our cases show that MR imaging can play a role during acute events in these patients and that sequences such as ASL and MRA should be included in the imaging protocol.
Our data corroborate the single observation of Almuqbil et al,16 who reported a 9-year-old child affected by GLUT1-DS with reversible attenuation of the distal branches of right MCA on MRA and ipsilateral reduced perfusion on ASL during acute-onset left hemiparesis and altered mental status.
Proton MRS was only performed in 1 case (case 2) during the acute phase and demonstrated no evidence of a lactate peak (Online Supplemental Data). Meanwhile, ADC values remained normal as in all other cases. One interpretation is that despite the unequivocal blood flow reduction, any possible transient cell energy dysfunction or failure (reduced EEG activity) may have remained below the decompensation threshold (cytotoxic edema) detectable by ADC mapping. Whether unilateral hypoperfusion was the cause or the epiphenomenon of transient cerebral functional impairment remains to be established.
Given that GLUT1 is predominantly expressed by the endothelial cells of the BBB and astrocytes, it can be hypothesized that acute cortical hypometabolism, resulting from inadequate glucose supply and consequent decrease in regional cerebral perfusion (neurovascular coupling), underpins these acute episodes. The detrimental effects of neuroglycopenia, similar to cellular “starvation,” may act on the endothelium and nervous tissue, altered by the transporter deficiency.
One of the hypotheses is that the vasospasm-like events in patients with GLUT1-DS are similar to those observed during the acute phase of hemiplegic migraine, which shares similar symptom patterns17 and in which acute vasospasm has also been occasionally reported on MRA.18 The etiology of hemiplegic migraine is not completely understood, but 1 prevailing theory suggests that a depolarization wave during the aura phase triggers cerebral perfusion changes, initially causing hypoperfusion followed by hyperperfusion, potentially resulting in damage to the BBB.19 Consistent EEG findings were observed in patients with hemiplegic migraine and GLUT1-DS during episodes of acute transient neurologic symptoms, characterized by contralateral EEG slowing.20 The phenomenon of spreading depression, hypothesized to precipitate migraine attacks, is typified by a widespread depolarization propagating through the cortex, leading to suppression of neuronal electrical activity. The elevation of extracellular potassium ions (K+) is believed to be associated with the depolarization of neighboring cells.21 Perfusion imaging of migraine attacks accompanied by an aura demonstrates a distinct pattern: an initial phase of hypoperfusion during the aura period, followed by hyperperfusion in regions extending beyond the boundaries of individual vascular territories.22 It is conceivable that a similar mechanism may underlie the observations documented in patients diagnosed with GLUT1-DS during acute events. Nevertheless, elucidating the precise trigger for spreading depression in these individuals poses a challenge.
Furthermore, the transient increased conspicuity of vein depiction observed with SWI in 2 cases has no univocal explanation. This finding has also been reported to be associated occasionally with migraine attacks.23 It is not currently clear whether it may represent a reactive venous side vascular dilation consequent to arterial bed reduction or an increased oxygen extraction with consequent deoxyhemoglobin production. The cases also showed some transient contralateral cerebellar hypoperfusion suggestive of a functional crossed cerebellar diaschisis phenomenon. It is commonly defined as a reduction in neuronal activity in the cerebellar hemisphere contralateral to a cerebral hemisphere affected by a lesion.24 Cerebellar hypoperfusion was transient as supratentorial flow changes. Crossed cerebellar diaschisis was more pronounced in case 1; notably, this patient was also the one who presented with cerebellar signs such as ataxia and dysmetria and also had the most symptom-resolution delay (Online Supplemental Data). In case 2, the difference in cerebellar hemisphere perfusion was milder, and there was a significant variation in perfusion levels across both cerebellar cortices during follow-up. Case 4, which lacked a follow-up study, seemed to exhibit a mild crossed cerebellar diaschisis during the acute phase. In case 3, mild crossed cerebellar diaschisis was evident in the presence of a gliotic-atrophic area in the ipsilateral cerebellar hemisphere. The patient lacked a documented history of prior stroke-like incidents; however, she exhibited a documented history of epileptic events. Hypothetically, this scenario could be interpreted as the permanent sequelae of multiple previous crossed cerebellar diaschisis episodes.
Transient functional crossed cerebellar diaschisis has also been reported in a small fraction of patients with migraine during the acute phase such as in the article by Kellner-Weldon et al,25 in which a cohort of patients was scrutinized during migraine episodes using MR perfusion imaging with a dynamic susceptibility contrast technique, unveiling oligemia in a cerebral hemisphere in 23 of 106 patients, most of whom manifested an aura. Cerebellar hypoperfusion was evident in 12 of the 23 patients. While crossed cerebellar diaschisis was documented in 9 of the 23 patients with oligemia, none of their patients presented with DWI restriction. It is possible that the 2 conditions may share some similar pathophysiologic aspects regarding the functional consequences on cerebellar status of defaulting supratentorial structures.
Moreover, all our patients had left cerebral hypoperfusion at presentation, and all were right-handed with left-sided hemispheric dominance. While this feature could be happenstance related to the small sample size, it is possible that the left hemisphere might be more at risk, perhaps due to higher energy demand. Observations in a larger number of cases would be needed to address this issue.
Morphologic and functional observations in patients with stroke-like episodes and GLUT1-DS are scant. Other case reports have primarily centered on clinical findings, in which the reported features were generally like those in our series: acute onset of hemiplegia, ataxia, speech disorders, and facial nerve deficit.26⇓-28 The use of some functional techniques such as perfusion assessment by ASL matched with water diffusion imaging, MRA, and EEG data may add some insight into the pathophysiology of this rare and intriguing condition.
Limitations
One of the inherent drawbacks our study is that it consists of a limited case series of patients with clear-cut acute stroke-like symptoms, so it might not be representative of the general population of patients with GLUT1-DS. There may have been bias in selecting the subset of patients with GLUT1-DS who experienced stroke-like symptoms. Moreover, smaller possible variations among subjects in this cohort may have been obscured by the different time-frames in which acute evaluation and follow-up were conducted. While ASL-based perfusion imaging allows imaging without the need for contrast agents, setting the appropriate parameters such as postlabel delay can be challenging, depending on the patient’s age. Different parameters apply to neonates, children, and adults respectively.29 Moreover, when using ASL, one must be aware of artifacts resulting from low cardiac output or vessel stenosis (which can cause arterial transit artifacts), the “ring of fire” caused by the labeling of CSF water protons, the shunt effect in arteriovenous malformations, and motion artifacts.30 Therefore, we recommend minimizing potential artifacts and hazards when using this perfusion approach, selecting the appropriate postlabel delay for the respective age group, and performing MRA to evaluate arterial tortuosity.
CONCLUSIONS
Drawing from our data and supported by a thorough review of the literature, we propose that hypoperfusion in the cerebral hemisphere and reduction in the representation of distal branches of the MCA in association with crossed cerebellar diaschisis may be more common in this patient population than previously recognized. The parallels observed between patients with hemiplegic migraine and GLUT1-DS experiencing acute stroke-like events could imply some shared pathophysiology. Further cohort studies are needed to extend our observations.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received May 4, 2024.
- Accepted after revision August 12, 2024.
- © 2025 by American Journal of Neuroradiology