Abstract
BACKGROUND AND PURPOSE: Contrast-enhanced MRI (CEMRI) is a commonly used imaging technique for craniopharyngioma surveillance; however, it carries risks such as allergic reaction and gadolinium deposition. This study evaluates the efficacy of non contrast–enhanced MRI (NCMRI) with a balanced steady-state free precession (bSSFP) sequence compared with CEMRI T1-weighted imaging for craniopharyngioma surveillance.
MATERIALS AND METHODS: Twenty-nine patients with craniopharyngioma (16 females/13 males, mean age =21.5 ± 4.3 years) with CEMRIs, including a bSSFP sequence, were evaluated. For each patient, 2 blinded neuroradiologists compared the dimensions of residual craniopharyngioma on non-contrast– and contrast-enhanced sequences. Tumor volume and solid/cystic component measurements were evaluated by using paired t-tests. Diagnostic confidence levels for non-contrast– and contrast-enhanced evaluations were measured by using a 3-point scale (2 = confident, 1 = adequate, 0 = unsure). Analyses of tumor involvement of cranial nerves (CNs) and adjacent vasculature and diagnostic confidence were performed by using Fisher exact and chi-square tests.
RESULTS: No significant difference was observed between residual tumor volumes in both studies (18.86 ± 21.67 cm3 versus 17.64 ± 23.85 cm3, P = .55) and measurements of dominant solid component volume, number of cystic components, and largest cystic component volume (2.71 ± 3.47 cm3 versus 3.95 ± 5.51 cm3, P = .10; 2.5 ± 1.5 versus 2.9 ± 1.5, P = .10; 7.61 ± 13.41 versus 6.84 ± 13.37 cm3, P = .22, respectively). Tumor involvement of CNs II (P = .64), III (P = .42), and adjacent vasculature (P = .05) showed no significant differences in detection. Diagnostic confidence was comparable in evaluating CN II, vascular structures, and third ventricle (P > .05) involvement. Higher levels of confidence were observed with bSSFP sequences for the detection of CN III involvement (P = .0001) and with contrast-enhanced T1-weighted imaging for cavernous sinus involvement (P = .02).
CONCLUSIONS: NCMRI techniques by using a bSSFP sequence provide similar characterization of craniopharyngiomas as contrast-enhanced techniques.
ABBREVIATIONS:
- ACA
- anterior cerebral artery
- bSSFP
- balanced steady-state free precession
- CEMRI
- contrast-enhanced MRI
- ce-T1W
- contrast-enhanced T1-weighted imaging
- CN
- cranial nerve
- NCMRI
- non-contrast–enhanced MRI
- TR
- total resection
SUMMARY
PREVIOUS LITERATURE:
Craniopharyngiomas are rare, benign brain tumors accounting for 1%-3% of all brain neoplasms. CEMRI is the standard imaging protocol for detecting and monitoring the tumor. Posttreatment, patients undergo frequent ce-T1W for tumor surveillance. Repeated exposure to gadolinium-based contrast can risk accumulation of free gadolinium in the brain parenchyma, which may have an impact on patients’ neurologic function. The use of a bSSFP sequence has shown promise as an alternative to standard brain tumor surveillance. To date, our study is the first to evaluate the efficacy of bSSFP in craniopharyngioma surveillance in comparison to the conventional ce-T1W.
KEY FINDINGS:
Our study found that the bSSFP sequence performed comparably to ce-T1W for assessing the presence of residual craniopharyngioma and for its involvement of surrounding structures. Similar diagnostic confidence was reported between the 2 sequences. bSSFP also provided higher diagnostic confidence for tumor involvement of CN III.
KNOWLEDGE ADVANCEMENT:
Our study suggests that bSSFP can serve as an alternative for craniopharyngioma surveillance because of its high diagnostic accuracy, cost-effectiveness, and lack of gadolinium exposure. The sequence can be especially useful for monitoring craniopharyngioma in pediatric patients and patients with impaired renal function.
Craniopharyngiomas are rare, benign brain neoplasms that account for approximately 1%–3% of all brain tumors.1 They originate from residual embryonic tissue within the squamous epithelium of the primitive pharynx and are typically found in the sella and suprasellar regions of the brain.2 Contrast-enhanced MRI (CEMRI) is the standard protocol for imaging craniopharyngiomas at both baseline detection and for continued surveillance both during and after treatment.3 As such, patients with craniopharyngiomas undergo frequent contrast-enhanced T1-weighted imaging (ce-T1W). The current protocol for imaging surveillance following the initiation of radiation therapy is a CEMRI every 3 months for the first year, followed by intervals of every 4–6 months up to 2 years, transitioning to annual scans thereafter.4
Recent studies have shown that free gadolinium can accumulate in the brain parenchyma, raising concerns about the safety of recurrent contrast use.5,6 These findings have prompted health care professionals to exercise increased caution when ordering contrasted studies, particularly since the long-term impact of gadolinium deposition remains unknown. This is especially relevant for pediatric patients who undergo repeated exposure to contrast for tumor surveillance.7
MR cisternographic imaging has emerged as an alternative approach for brain tumor surveillance. A balanced steady-state free precession (bSSFP) sequence (also known as CISS, FIESTA, or 3D driven equilibrium) is a heavily fluid-weighted sequence with high spatial resolution that has been shown to be effective in evaluating cranial nerves (CNs) and cisterns.8,9 Previous work has demonstrated the effectiveness of bSSFP for evaluation and surveillance of various brain tumor types in comparison to conventional contrast-enhanced MRI.9⇓–11 A notable advantage of the bSSFP protocol is its independence from gadolinium-based contrast, thereby eliminating the risk of contrast deposition and making it more suitable for long-term surveillance.
In this study, we aimed to assess the diagnostic efficacy of a non-contrast–enhanced MRI (NCMRI) protocol utilizing the bSSFP sequence for craniopharyngioma surveillance in comparison to conventional CEMRI examinations.
MATERIALS AND METHODS
Patient Cohort
This was a retrospective study performed at a single institution that was approved by the Institutional Review Board and was compliant with the Health Insurance Portability and Accountability Act. A total of 29 patients who had a pathology-proven sellar/suprasellar craniopharyngioma that underwent primary surgical resection between 2008 and 2018 were included in the study. Each patient underwent brain MRI examination with both bSSFP and conventional ce-T1W sequences between 3 to 6 months after surgery.
MRI Protocol
All patients underwent the same MRI protocol, consisting of axial T2-weighted imaging, axial FLAIR, axial susceptibility-weighted imaging, axial DWI (including ADC reconstruction from the DWI), precontrast T1-weighted imaging, ce-T1W, and bSSFP. All brain MR examinations were performed on 3T scanners at our institution (Magnetom Prisma Fit and Magnetom Skyra, Siemens Healthineers).
The ce-T1W and bSSFP sequences were used for image evaluation. The parameters of these sequences are as follows: ce-T1W – TR 14.764 ms, TE 6.364 ms, flip angle 13°, NEX 1, FOV 260 × 260, matrix 0\260\260\0; bSSFP – TR 5.832 ms, TE 2.192 ms, flip angle 65°, NEX 4, FOV 180 × 180, matrix 0\448\256\0. The duration of the bSSFP sequence was 7:29 minutes.
Image Evaluation
Two board-certified neuroradiologists, blinded to all other imaging and clinical information, evaluated each patient’s brain MRI examinations in 2 rounds separated by greater than 2 weeks. For the first-round evaluation, only NCMRI sequences, including the bSSFP sequence, were evaluated. The second-round evaluation utilized postcontrast sequences, excluding the bSSFP sequence. This technique of 2 rounds of imaging-based assessment is in line with techniques previously published in the literature.9,10 The neuroradiologists evaluated the images together to reach a consensus. The order of the patients was randomized for each evaluation session.
The axial, transverse, and craniocaudal dimensions of the residual craniopharyngioma were determined for both sequences. The neuroradiologists were asked to assess their diagnostic confidence for the tumoral involvement of the adjacent structures in each study by using a 3-point scale (2 = confident, 1 = adequate, 0 = unsure). This assessment was done for CN II, CN III, cavernous sinus, middle cerebral artery (MCA) anterior cerebral artery (ACA), basilar artery, internal carotid artery (ICA) and the third ventricle.
Statistical Analysis
Tumor volume, as well as solid and cystic component measurements, were evaluated by using paired t-tests. The analysis of diagnostic confidence for tumoral involvement of the adjacent structures was performed by using chi-square and Fisher exact tests.
RESULTS
Patient Demographics
A total of 29 patients were included in this study (Table 1), with 13 (44.8%) males and 16 (55.2%) females. The mean age was 21.5 ± 4.3 years. Approximately 21 (72.4%) patients exhibited the adamantinomatous type of craniopharyngioma, while the remaining 8 (27.6%) patients had an unspecified type. Most of the patients (27, 93.1%) underwent subtotal resection; meanwhile, gross total resection was reported in 2 (6.9%) patients.
Demographics
Evaluation of Tumoral Size and Involvement
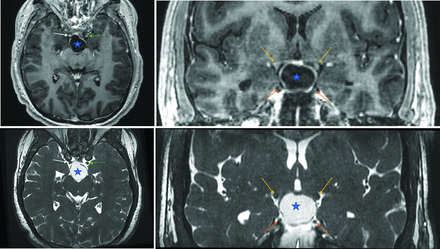
No statistically significant difference was observed between residual tumor volume measured on bSSFP and ce-T1W sequences (18.86 ± 21.67 cm3 versus 17.64 ± 23.85 cm3, P = .55; Table 2). Similarly, measurements of the dominant solid component volume, the number of cystic components, and the largest cystic component volume demonstrated no significant differences between the 2 sequences (2.71 ± 3.47 cm3 versus 3.95 ± 5.51 cm3, P = .10; 2.5 ± 1.5 cm3 versus 2.9 ± 1.5 cm3, P = .10; 7.61 ± 13.41 cm3 versus 6.84 ± 13.37 cm3, P = .22, respectively). Furthermore, the tumoral involvement of CN II (P = .64), CN III (P = .42), and the adjacent vasculature (P = .05) showed no significant differences. Figures 1 and 2 show 2 representative craniopharyngioma cases where ce-T1W and bSSFP sequences similarly evaluate the tumor and its surrounding structures.
Axial postcontrast T1-weighted (top left) and bSSFP (bottom left) images demonstrate a suprasellar predominantly cystic craniopharyngioma (star). Both sequences demonstrate abutment of the right internal carotid artery by the craniopharyngioma (white arrows). The left internal carotid artery (green arrow) is spared. In the coronal view, both postcontrast T1-weighted (top right) and bSSFP (bottom right) images adequately show the relationship between the craniopharyngioma and the optic tract (yellow arrows) and CN III (orange arrows), which are not in direct contact.
Axial postcontrast T1-weighted (top left) and bSSFP (bottom left) images demonstrate a suprasellar mixed cystic/solid craniopharyngioma (star). Both sequences demonstrate that the lesion abuts the optic chiasm anteriorly (yellow arrow), with splaying of the optic tracts (blue arrow) along the lateral aspects of the craniopharyngioma. Posteriorly, the craniopharyngioma abuts the mammillary bodies (green arrows). In the coronal view, both postcontrast T1-weighted (top right) and bSSFP images (bottom right) demonstrate how the craniopharyngioma is encroaching on, but not in direct contact with, the A1 segments of the anterior cerebral arteries (orange arrows).
Discrepancies in measurements between CEMRI and bSSFP
Interrater Reliability
The diagnostic confidence was comparable for evaluation of adjacent structures in both studies (Table 3). There was no statistically significant difference in the diagnostic confidence for tumor involvement of CN II, MCA, ACA, basilar artery, and the third ventricle between bSSFP and ce-T1W (93.1 versus 96.6, P = .55; 100 versus 96.6, P = .31; 100 versus 96.6, P = .31; 100 versus 96.6, P = .31; 93.1 versus 89.7, P = .64; 100 versus 93.1, P = .15, respectively). Interestingly, a significantly higher level of confidence was observed when bSSFP was used to detect CN III involvement (P = .0001) and ce-T1W for cavernous sinus involvement (P = .02).
Comparative assessment of diagnostic confidence for tumor involvement in adjacent structures
DISCUSSION
Although the diagnostic utility of bSSFP sequences for the evaluation and surveillance of skull base tumors has been well-established, its use in craniopharyngioma surveillance has yet to be described. The findings of our study suggest that bSSFP performs comparably to CEMRI for the assessment of craniopharyngiomas after treatment. Given its superior capability in distinguishing between soft tissue and CSF within the basal cisterns and ventricular system, along with its high signal-to-noise ratio, bSSFP was able to effectively differentiate residual craniopharyngioma from the surrounding CSF, particularly in the suprasellar region where craniopharyngiomas are commonly found.
Additionally, a bSSFP sequence provides better diagnostic confidence in detecting the involvement of CN III by residual craniopharyngioma. The improved confidence and accuracy in assessing tumor involvement can be important in optimizing treatment strategies and minimizing potential complications.12
Our observations align with the findings of a previous study that compared the diagnostic confidence in detecting residual germ cell tumors when bSSFP and ce-T1W sequences were used.9 With its improved contrast and high spatial resolution, bSSFP was able to provide better detection of the tumor in the pineal and suprasellar regions where there is a high volume of CSF.9 Moreover, this study indicated that the use of bSSFP can improve diagnostic confidence when assessing the presence of residual tumor.9 An improvement in interrater agreement was also reported with the use of bSSFP (0.872 with bSSFP and 0.128 without bSSFP). Interestingly, the study found that bSSFP can detect small tumor volumes that may be overlooked by ce-T1W.9 A bSSFP sequence has also been shown to have similar or increased effectiveness for surveillance of other types of brain tumors, such as for the detection of spinal drop metastases in pediatric patients.10
Cyst enlargement is a prevalent complication, which can present both during and after radiation therapy and surgical resection. It may potentially result in obstructive hydrocephalus and visual deficits secondary to optic nerve compression.13,14 Given the observed superiority of the bSSFP sequence in assessing the cystic component of tumors, bSSFP can facilitate the early recognition and detection of such complications, which allows proper and timely interventions that improve patient outcomes.
A bSSFP sequence offers additional advantages compared with traditional ce-T1W. These include improved cost-effectiveness, feasibility, and a reduced risk of potential negative health outcomes due to gadolinium deposition. A bSSFP sequence can be considered more cost-effective since intravenous contrast administration is not used. Furthermore, prolonged use of gadolinium-based contrast may risk free gadolinium deposition in brain tissue and increase the risk of nephrogenic systemic fibrosis, especially in patients with reduced renal function or pediatric patients.15 Additionally, bSSFP is considered more feasible for pediatric patients as obtaining intravenous access can be difficult.
Given the effectiveness and advantages of the bSSFP sequence with its superior spatial and contrast resolution, adopting a noncontrast protocol for craniopharyngioma surveillance is highly recommended, especially for pediatric patients and patients with impaired renal function. Because of the similar performance of bSSFP and ce-T1W for evaluating craniopharyngiomas, the use of both sequences may not be necessary for some patients. While some patients may benefit from the use of both imaging techniques for tumor surveillance, our results suggest that performing only 1 of the 2 sequences can be sufficient for tumor surveillance.
This study’s limitations include the small sample size, the lack of long-term follow-up, and the retrospective nature of the study. Patients included in this study were 3–6 months postsurgery, and thus, the follow-up window was relatively short. More information and a more detailed study are needed to fully investigate the use of the bSSFP sequence for long-term follow-up of recurrent or residual tumors. Furthermore, some cases were excluded because of missing information. A prospective study should be conducted in the future to better compare posttreatment tumor surveillance between the 2 imaging techniques. However, our results provide valuable insight into the optimization of imaging protocols for craniopharyngioma surveillance. Further research with a larger cohort is warranted to validate the efficacy of the bSSFP sequence in routine practice.
CONCLUSIONS
This study suggests that NCMRI, by using a bSSFP sequence, is comparable to ce-T1W MRI for craniopharyngioma surveillance. The potential advantages include reduced contrast exposure and improved diagnostic confidence for CN III involvement.
Footnotes
Kelly Trinh and Michael Tang contributed equally to this article.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received April 27, 2024.
- Accepted after revision July 19, 2024.
- © 2025 by American Journal of Neuroradiology