Abstract
BACKGROUND: Brain tumors are a leading cause of mortality in children. Accurate tumor grading is essential to plan treatment and for prognostication. Perfusion imaging has been shown to correlate well with tumor grade in adults, however there are fewer studies in pediatric patients. Moreover, there is no consensus regarding which MR perfusion technique demonstrates the highest accuracy in the latter population.
PURPOSE: We sought to compare the diagnostic test accuracy of DSC and arterial spin-labeling (ASL), in their ability to differentiate between low- and high-grade pediatric brain tumors at first presentation.
DATA SOURCES: Articles were retrieved from online electronic databases: MEDLINE (Ovid), Web of Science Core Collection, and Scopus.
STUDY SELECTION: Studies in pediatric patients with a treatment-naïve diagnosed brain tumor and imaging including either ASL or DSC or both, together with a histologic diagnosis were included. Studies involving adult patient or mixed age populations, studies with incomplete data, and those that used dynamic contrast-enhanced perfusion were excluded.
DATA ANALYSIS: The sensitivities and specificities obtained from each study were used to calculate the true-positive, true-negative, false-positive, and false-negative count. A case was defined as a histologically proved high-grade tumor. The random-effect model was used to merge statistics. Significance level was set at P < .05.
DATA SYNTHESIS: Forest plots showing pairs of sensitivity and specificity, with their 95% CIs, were constructed for each study. The bivariate model was applied to account for between-study variability. The summary receiver operating characteristics (SROC) plots were constructed from the obtained data sets. The area under the curve for the SROC of all studies was estimated to determine the overall diagnostic test accuracy of perfusion MRI, followed by a separate comparison of the SROC of ASL versus DSC studies.
LIMITATIONS: There was a small and heterogeneous sample size.
CONCLUSIONS: The diagnostic accuracy of ASL was found to be comparable and not inferior to DSC, thus its use in the diagnostic assessment of pediatric patients should continue to be supported.
ABBREVIATIONS:
- ASL
- arterial spin-labeling
- AUC
- area under the curve
- DCE
- dynamic contrast-enhanced
- HG
- high-grade
- LG
- low-grade
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QUADAS-2
- Quality Assessment of Diagnostic Accuracy Studies 2
- rCBF
- relative CBF
- rCBV
- relative CBV
- SROC
- summary receiver operating characteristic
- WHO
- World Health Organization
SUMMARY
PREVIOUS LITERATURE:
MR perfusion imaging has been shown to correlate well with brain tumor grade in adults, however there are fewer studies in pediatric patients. Moreover, there is no consensus regarding which perfusion technique demonstrates the highest accuracy in the latter population. Whilst in the past DSC has been used more frequently than ASL in the evaluation of brain tumor grade in pediatric patients, ASL is a non-invasive technique with additional advantages making it a more favorable option in this population.
KEY FINDINGS:
This meta-analysis confirms that the application of ASL and DSC perfusion MRI confers a good diagnostic accuracy in distinguishing between low- and high-grade brain tumors in the pediatric population, with an AUC estimated at 0.866. From our results it appears that ASL seems to perform better, with a slightly higher pooled sensitivity than for DSC and with both giving the same pooled false positive rate. However, the difference between the two sensitivities was not statistically significant.
KNOWLEDGE ADVANCEMENT:
As the diagnostic accuracy of ASL has been shown to be comparable and not inferior to DSC, its use in the diagnostic assessment of these patients should continue to be supported.
Brain tumors are the most common solid tumors in the pediatric population with an incidence of 6.14 per 100,000. They are also a leading cause of mortality in this age group, surpassing other cancers and recognized as the top reason for cancer mortality in those aged between 0 and 14 years at diagnosis.1,2 Accurate grading of these tumors before treatment is clinically important because it allows for appropriate planning of therapeutic approach and prognostication. While the current diagnostic standard is histopathology from biopsy or surgical resection, there are a number of cases in which surgical access is not feasible or carries high risk. Imaging plays a major role in diagnosis, surgical planning, and assessment following treatment. Conventional brain MRI in isolation is often limited in this regard, and often fails to provide sufficient diagnostic test accuracy regarding underlying tumor biology.3,4 The clinical necessity for an imaging-based assessment of tumor grade in the field of neuro-oncology has led to the development of advanced MRI techniques. Of the modalities providing information on the physiology of tumors, perfusion MRI is a technique aimed at assessing hemodynamic parameters, providing quantitative maps of CBF, CBV, and MTT together with vascular permeability parameters.5
MRI perfusion has become increasingly relevant in brain tumor assessment, given the established use of antiangiogenic and antivascular therapies6 as well as for its ability to provide information regarding long-term survival7 and tumor grade. Traditionally, DSC and arterial spin-labeling (ASL), the most widely used techniques, have been believed to have complementary roles in perfusion imaging of brain tumors, with ASL on the one hand being more sensitive to absolute quantification of tumor blood flow, and DSC on the other hand being more sensitive to alterations in tissue permeability and capillary blood volume. Nevertheless, a comparison of their respective ability to assess tumor grade remains a valid research topic due to a number of clinically relevant differences between these techniques.
ASL is a completely noninvasive technique and does not require the administration of gadolinium-based contrast agents. This makes it ideal for scenarios in which contrast is contraindicated or best avoided, such as in renal impairment, history of anaphylactic reactions against contrast, and pediatric patients in general. This is especially relevant in light of the evidence that administration of contrast results in accumulation of gadolinium in the brain, even in patients without severe renal impairment.8,9 It is also useful where venous access is difficult or not feasible, as is oftentimes the case in young children, particularly those undergoing chemotherapy. It additionally aids in scenarios in which repeated examinations are necessary, such as in cases of failed sedation or patient motion. On the other hand, DSC relies on reasonably high contrast doses, particularly when correcting for leakage effects, and requires the use of a high-flow power injector and large caliber venous access, posing considerable technical challenges in young patients. Bolus delay and dispersion caused by slow injection rates may lead to underestimation of CBF values. Furthermore, in the case of an inadequate study as is quite frequent in young children, it is not possible to repeat DSC in the same examination without a further bolus administration of contrast agent. ASL on the other hand does not require leakage calculation and correction.10
As a technique, DSC is extremely vulnerable to image distortion and susceptibility artifacts from interfaces of brain with bone or air, and those resulting from the presence of blood products and calcium.11 In ASL, susceptibility effects causing signal drop-out and geometric distortions are comparatively less prominent because shorter echo times are used. It may therefore be better suited in the evaluation of pediatric brain tumors adjacent to the skull base. Because pediatric patients possess a higher cerebral water content and, overall, more CBF than adults, a higher SNR and reduction in artifacts can be afforded in ASL studies in this population.12
While these imaging techniques have been well evaluated in adult patients, with a number of studies demonstrating equivalent diagnostic test accuracy of ASL compared with DSC, in terms of distinction between low-grade (LG) and high-grade (HG) tumors as well as in prognostication,13⇓⇓⇓⇓⇓⇓–20 there is less targeted research specifically relating to pediatric patients and no clear consensus of the clinical role of these techniques. This is especially relevant when considering that the biologic features of pediatric brain tumors are unique. The recently published 2021 update of the World Health Organization (WHO) Classification of Tumors of the Central Nervous System,21 includes a number of notable changes, whereby the differences between pediatric and adult brain tumors are being increasingly recognized, compelling the undertaking of targeted research in this regard.
The hypothesis underlying our study is that ASL perfusion is comparable to DSC perfusion in its ability to distinguish between LG and HG brain tumors in the pediatric population. The scope was to assess if there is a significant difference in the diagnostic test accuracy of DSC and ASL, in their ability to differentiate between LG and HG pediatric brain tumors at first presentation.
MATERIALS AND METHODS
Criteria for Considering Studies for Inclusion and Search Strategy
The Patients, Interventions, Comparisons and Outcomes model was used to define the research question for this study.22 A summary of the inclusion and exclusion criteria is provided in Table 1. This metanalysis was undertaken in accordance with the Cochrane Handbook for Diagnostic Test Accuracy Reviews.23 The Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 guidelines24 were also followed. A systematic search strategy for quantitative data literature was developed. Articles were retrieved from MEDLINE (Ovid), Web of Science Core Collection, and Scopus (Online Supplemental Data). The search only considered human studies and was limited to studies in the last 10 years. The most recent search for this review was run on August 5, 2022.
Inclusion and exclusion criteria
Selection of Studies
Retrieved hits were transferred onto an online systematic review screening tool.25 After the removal of duplicates, the remaining articles were assessed for inclusion independently by 2 researchers: a consultant and resident specialist in radiology with subspecialization in neuroradiology, with 10 years and 1 year of experience in meta-analyses, respectively. In addition, the references from chosen articles were manually reviewed with the aim of identifying any further potentially relevant studies that were not detected in the initial search. The flow chart of retrieval process is presented in Fig 1. After full-text evaluation, 51 studies were excluded for the following reasons. Eighteen records were review articles or meta-analyses lacking original quantitative data. Nine studies did not consider tumor grading but rather disease progression, overall survival, or aimed to assess the technical aspects of the perfusion techniques. In 7 of the reports the study population was adult, while 9 of the studies included both adult and pediatric patients with no possibility of separating the respective results. Finally, in 7 of the remaining studies, quantitative data values necessary for the statistical analysis, including the sensitivities and specificities obtained, were not reported. The respective authors were contacted and requested to provide this information; however, no response was received. Ten studies that included a total of 477 patients were included in the meta-analysis.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart of the study inclusion process.
Data Extraction
The following data were extracted independently by two researchers from each of the included studies onto a pre-set sheet:
General Information: Study title, first author, journal, country of origin, year of publication, and study design (prospective versus retrospective).
Patient Information: Sample size, age range, type of brain tumors being studied, and tumor WHO grade.
Imaging Information: MR scanner model, manufacturer and field strength, method of MR perfusion performed, ASL technique (pseudocontinuous or pulsed), DSC bolus regimen, imaging parameters including TR, TE, slice thickness, matrix size, field of view, flip angle, labeling duration and postlabeling delay (the latter in the case of ASL), ROI evaluation technique, and reference region used.
Quantitative Results: Data regarding relative CBV (rCBV), relative CBF (rCBF), sensitivity, specificity, summary receiver operating characteristic (SROC) curve with the corresponding area under the curve (AUC) value, were extracted based on authors' prespecified and recommended thresholds.
Critical Appraisal Tool
Each of the included studies was critically appraised based on the revised Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool.26 Two review authors independently extracted information from each study based on the published articles and any available supplementary material. An adapted template created by the authors of a previously published metanalysis was used in the assessment of included studies (Online Supplemental Data).27 Any incongruities were dealt with through discussion with a third senior clinician; a consultant radiologist with subspecialization in neuroradiology with 7 years’ experience in meta-analyses.
Data Analysis
All analyses were performed using R Statistical Software (v4.2.2 Patched [2022-11-10 r83330]).28 The sensitivities and specificities obtained from each study were used to calculate the true-positive, true-negative, false-positive, and false-negative count. A case was defined as a histologically proven HG tumor. A positive index test implied a diagnosis of an HG tumor, while a negative test suggested an LG tumor. Thus, true-positives were correctly diagnosed as HG tumors, false-negatives were HG tumors incorrectly labelled as LG tumors, true-negatives were correctly diagnosed as LG tumors, while false-positives were LG tumors incorrectly labeled as HG tumors. Between-study heterogeneity variance was measured by τ2 and I2 values. The random-effect model was used to merge statistics. Significance level was set at P < .05. Forest plots showing pairs of sensitivity and specificity, with their 95% CIs, were constructed for each study using metafor.29
The bivariate model30 was applied to account for between‐study variability in estimates of sensitivity and specificity through the inclusion of random effects for the logit sensitivity and logit specificity parameters of the bivariate model. The reitsma function of the mada package was used to generate the bivariate model parameters required to construct the SROC plot from the obtained data sets.31 The AUC for the SROC of all studies was estimated to determine the overall diagnostic test accuracy of perfusion MRI, followed by a separate comparison of the SROC of ASL versus DSC studies.
RESULTS
The data of the included literature is presented in Table 232,–,41 and the Online Supplemental Data. Collectively, the 10 studies included in this meta-analysis reported 477 patients with brain tumors. All studies were retrospective and avoided case‐control design. The sample size per study was generally adequate and ranged from 19 to 117 cases. Only 1 study had a sample size of less than 20.36 The following tumor types were assessed across all studies: gliomas and glioneuronal tumors (n = 304), ependymomas (n = 41), embryonal (n = 115), hemangioblastoma (n = 1), germ cell (n = 1), craniopharyngioma (n = 4), choroid plexus (n = 7), pineal (n = 3), chordoma (n = 1). All cases but 1 diffuse midline glioma received histologic diagnosis. Hence, the bulk of the tumors were of the glial and embryonal groups, which reflects their incidence. Most of the included studies used the 2016 WHO brain tumor classification. DSC was used in 3 of the included studies, while ASL was the perfusion technique assessed in 5 studies. Two of the included studies used both DSC and ASL. In 4 of the ASL studies, the pulsed technique was used, with the remaining being pseudocontinuous. None of the DSC studies used contrast agent preloading. Information regarding arterial input function was only available in 2 of the 5 DSC studies, where it was automated. Most examinations were performed on a 1.5T scanner (in 4 studies), 1 study used a 3T scanner, and 5 studies used both.
Characteristics of included studies
The methodologic quality assessment of each of the included studies (according to the modified QUADAS‐2 criteria) is summarized in Table 3. None of the included studies had low risk of bias and low concern for applicability across all domains, however all were deemed to be of eligible quality. The average rCBV/rCBF values per tumor grade in each included study are presented in Table 4.
QUADAS-2 methodologic quality assessment
Relative/normalized CBV and CBF values per tumor grade
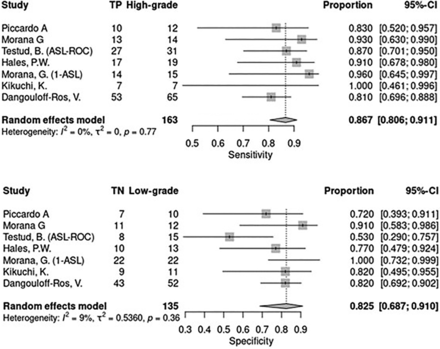
With regards to sensitivity of ASL, the between-study heterogeneity variance as measured by τ2 was negligible, indicating strong homogeneity between the studies. The pooled sensitivity was 0.867 with a 95% CI equal to 0.806–0.911. In terms of specificity of ASL, although the between-study heterogeneity variance was higher, as estimated by τ2 = 0.536 and I2 = 0.536, these figures were not statistically significant (P = .36). The pooled value of the specificity was 0.825 with a 95% CI = 0.687–0.910 (Fig 2).
Sensitivity and specificity plots for ASL.
In terms of sensitivity of DSC, I2 was noted to be high at 70%, indicating that more than one-half of the variability between the studies was not due to random fluctuations. The between-study heterogeneity variance was estimated at τ2 = 1.928. The pooled value of the sensitivity was 0.888 with a 95% CI = 0.638–0.973. As noted with ASL, the heterogeneity among the DSC studies was greater for specificity than for sensitivity, at least as measured by τ2 = 2.323. The I2 value was practically the same at 69%, with this variance among the studies again noted to be significant (P = .01). The pooled estimate of the specificity for these studies was 0.835 with a 95% CI = 0.512–0.960 (Fig 3).
Sensitivity and specificity plots for DSC.
Moderator analyses could not be performed due to an insufficient number of studies. In view of the overall substantial heterogeneity, a random-effects model was used to merge statistics. Meta‐analysis of the 10 studies by using a bivariate model reitsma function was run with its default method of restricted maximum likelihood (reml). The AUC for the SROC was estimated as 0.866. The point estimates for the pooled sensitivity and false-positive rates were 0.798 and 0.201, respectively (Fig 4). The Akaike information criterion for the 2 summary plots were −22.487 for ASL and −4.416 for DSC. This indicates that the ASL plot is a better fit of the data, which is expected given that DSC data comprised a smaller number of studies and also demonstrated greater heterogeneity (Fig 5). For ASL, pooled AUC = 0.876, pooled sensitivity = 0.824, 95% CI = 0.757–0.876, pooled false-positive rate = 0.204, and 95% CI = 0.142–0.285.
SROC curve (bivariate) for all studies.
Comparison SROC curves (bivariate) of ASL and DSC.
For DSC, pooled AUC = 0.861, pooled sensitivity = 0.789, 95% CI = 0.552–0.919, pooled false-positive rate = 0.203, and 95% CI = 0.081–0.425.
DISCUSSION
This meta-analysis confirms that the application of ASL and DSC perfusion MRI confers a good diagnostic accuracy in distinguishing between LG and HG brain tumors in the pediatric population, with an AUC estimated at 0.866. This is clinically relevant given that a preoperative indication of tumor grade is important to guide treatment decisions and strategies, as well as for prognostication.
DSC has been the traditionally used MR perfusion technique. LG tumors are generally less vascularized than HG tumors, therefore the hemodynamic parameters measured by DSC would be expected to be significantly increased in the latter. On analysis it was found that the pooled sensitivity and specificity of rCBV obtained by DSC to discriminate between LG and HG tumors was 78% and 80%, respectively. A limitation of this perfusion technique is the presence of leakage effects, the correction of which has led to a variety of approaches to obtain and process the obtained data. One such approach is the use of a contrast preload as has been discussed, however this is rarely done in children. The Boxerman consensus42 advocates the application of leakage correction in the postprocessing and provides guidance to that effect. The approach to leakage correction was not well described in our studies and when it was, did vary somewhat across the studies that were included.
Unlike DSC, perfusion measurements in ASL are not tampered by blood-brain barrier permeability effects. Furthermore, because shorter echo times are used, susceptibility effects leading to signal drop-out and geometric distortions are less frequently encountered. ASL does, however, suffer from low SNR and motion sensitivity. Additionally, measurement of absolute CBF is known to be unreliable due to patient variables, although, even when relative CBF is calculated, variation is still possible due to the difference in pulse sequences and post processing algorithms that vary between centers. Recently published consensus guidance regarding the most appropriate implementation for clinical applications and imaging parameters43 should standardize practice, however several of the included studies comprised cases that preceded these recommendations, also possibly accounting for a proportion of the heterogeneity that was noted. On analysis it was found that the pooled sensitivity and specificity of rCBF obtained by ASL to discriminate between LG and HG tumors was 82% and 80%, respectively.
There have been a number of studies comparing the performance of ASL and DSC in brain tumor grading in adults,13⇓⇓⇓⇓⇓⇓–20 however, given the different biology of pediatric brain tumors, extrapolation of adult study findings to this age group would be inappropriate. A recent study by Morana et al38 noted this lacuna in the pediatric population and demonstrated that ASL provides comparable results to DSC in pediatric astrocytic tumors, allowing distinction between LG and HG forms. A subsequent study noted moderate abilities of DSC and ASL to distinguish LG and HG pediatric brain tumors.40 There have not been any other such studies that directly compared ASL with DSC, however, a number of studies have assessed each technique independently. Through a thorough systematic review, it was aimed to extract the diagnostic data from various studies and assess if there is a significant difference in the performance of these 2 techniques. From our results it can be surmised that ASL seems to perform better, with a slightly higher pooled sensitivity than for DSC and with both giving the same pooled false-positive rate. However, the difference between the 2 sensitivities was not statistically significant. The confidence interval of the estimate for ASL contains the point estimate for DSC, and vice versa.
Substantial heterogeneity was noted in this study cohort. One reason for this could be the different tumor types and behaviors. In fact, the initial aim was to limit the analysis to assessment of pediatric gliomas. However, during our search it was noted that a number of studies included other brain tumor types, albeit in much smaller numbers. Gliomas did however account for most cases in our study population. Although the proportion of HG versus LG tumors was approximately equal in most studies, in 1 of the studies there were approximately double the number of HG tumors compared with LG.40 Additionally, field strengths, parameter settings, postprocessing methods, and ROI measurement method varied across studies. These factors most likely contributed to the variations noted, however, moderator analyses could not be performed due to an insufficient number of studies.
The relatively small sample size/included number of studies is both a reflection of the limited research in this field, and also of the strict methodology standards that were adhered to so as to remain faithful to the review question. Incomplete inclusion of data in publications and lack of author response also contributed. Nevertheless, neuro-oncologic imaging is a continuously evolving field and further research in this regard needs to be undertaken, preferably by using standardized scanning protocols to reduce heterogeneity. Harmonization of imaging and interpretation techniques is key, leading to clearer comparison between studies with the potential downstream effect of generating a higher level of evidence. Quality assessment revealed a high risk of bias in the index tests of 7 of our included studies, which we consider to be a further limitation.
Lastly, most of our studies defined the standard of tumor diagnostic assessment as histologic/immunohistochemistry grading. However, as highlighted in the recent WHO classification update, the biologic behavior and prognosis of brain tumors is becoming increasingly defined through molecular profiling. This modification will need to be considered and evaluated in future studies.
CONCLUSIONS
While DSC has been used more frequently than ASL in the evaluation of brain tumor grade in pediatric patients, the application of ASL is steadily increasing. ASL is a noninvasive technique and does not require the administration of gadolinium-based contrast agents. As the diagnostic accuracy of ASL has been shown to be comparable and not inferior to DSC, its use in the diagnostic assessment of these patients should continue to be supported.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received April 8, 2024.
- Accepted after revision July 26, 2024.
- © 2025 by American Journal of Neuroradiology