Abstract
BACKGROUND AND PURPOSE: Intracranial vessel wall imaging is technically challenging to implement, given the simultaneous requirements of high spatial resolution, excellent blood and CSF signal suppression, and clinically acceptable gradient times. Herein, we present our preliminary findings on the evaluation of a deep learning–optimized sequence using T1-weighted imaging.
MATERIALS AND METHODS: Clinical and optimized deep learning–based image reconstruction T1 3D Sampling Perfection with Application optimized Contrast using different flip angle Evolution (SPACE) were evaluated, comparing noncontrast sequences in 10 healthy controls and postcontrast sequences in 5 consecutive patients. Images were reviewed on a Likert-like scale by 4 fellowship-trained neuroradiologists. Scores (range, 1−4) were separately assigned for 11 vessel segments in terms of vessel wall and lumen delineation. Additionally, images were evaluated in terms of overall background noise, image sharpness, and homogeneous CSF signal. Segment-wise scores were compared using paired samples t tests.
RESULTS: The scan time for the clinical and deep learning–based image reconstruction sequences were 7:26 minutes and 5:23 minutes respectively. Deep learning–based image reconstruction images showed consistently higher wall signal and lumen visualization scores, with the differences being statistically significant in most vessel segments on both pre- and postcontrast images. Deep learning–based image reconstruction had lower background noise, higher image sharpness, and uniform CSF signal. Depiction of intracranial pathologies was better or similar on the deep learning–based image reconstruction.
CONCLUSIONS: Our preliminary findings suggest that deep learning–based image reconstruction–optimized intracranial vessel wall imaging sequences may be helpful in achieving shorter gradient times with improved vessel wall visualization and overall image quality. These improvements may help with wider adoption of intracranial vessel wall imaging in clinical practice and should be further validated on a larger cohort.
ABBREVIATIONS:
- ACA
- anterior cerebral artery
- AI
- artificial intelligence
- CAIPIRINHA
- Controlled Aliasing in Parallel Imaging Results in Higher Acceleration
- DL
- deep learning
- DLBIR
- deep learning–based image reconstruction
- IC-VWI
- intracranial vessel wall imaging
- PCA
- posterior cerebral artery
SUMMARY
PREVIOUS LITERATURE:
Intracranial vessel wall imaging is technically challenging, given the simultaneous requirement for ultra-high-resolution imaging, as well as achieving excellent CSF and blood flow suppression in clinically reasonable scan times, while preserving the signal from the vessel wall. In recent years, there has been increasing interest in use of artificial intelligence (AI) based techniques to enhance image quality in intracranial vessel wall imaging.
KEY FINDINGS:
Deep learning-based image reconstruction (DLBIR) T1 SPACE sequences images showed consistently higher wall signal and lumen visualization scores, with the differences being statistically significant in most vessel segments on both pre and post contrast images. DLBIR images had lower background noise, higher image sharpness and uniform CSF signal.
KNOWLEDGE ADVANCEMENT:
Deep learning-based reconstruction of 3D-T1-SPACE vessel wall imaging is helpful in achieving shorter gradient times with improved vessel wall visualization and overall image quality.
Intracranial vessel wall imaging (IC-VWI) has played an increasingly important role in routine neuroradiology practice in terms of detecting, characterizing, and differentiating various vasculopathies.1⇓⇓–4 IC-VWI is technically challenging, given the simultaneous requirement for ultra-high-resolution imaging as well as achieving excellent CSF and blood flow suppression in clinically reasonable scan times while preserving the signal from the vessel wall.2,5,6 Even though IC-VWI can be performed using either proton-density or fast spin-echo sequences using a variable flip angle refocusing pulse, a recent survey of the American Society of Neuroradiology membership noted that most responders predominantly used 3D sequences with T1-weighting (for example, Sampling Perfection with Application optimized Contrast using different flip angle Evolution (SPACE; Siemens), CUBE (GE Healthcare), or volume isotropic turbo spin-echo acquisition (VISTA; Philips Healthcare).2,4
Despite the enthusiasm and increasingly recognized role of IC-VWI in cerebrovascular pathologies, achieving clinically acceptable scan times while preserving image resolution remains a challenge. In terms of high spatial resolution in clinical practice, a voxel size of 0.5 mm isotropic is considered a reasonable starting point. However, most experienced centers use 3D acquisitions with isotropic voxels in the 0.4–0.7 mm range. At a voxel size of 0.5 mm isotropic, the circle of Willis and second-/third-order branches can be covered in about 7−10 minutes.2,6 The use of compressed sensing can help reduce scan times to about 7 minutes (or less, based on the acceleration factor) for whole-brain acquisition, albeit with a reduced contrast-to-noise ratio.7 Similarly, even though the use of parallel imaging such as Controlled Aliasing in Parallel Imaging Results in Higher Acceleration (CAIPIRINHA) sequences can help reduce scan times, the image quality is considerably inferior due to the intrinsic loss of signal-to-noise for conventional reconstructions.8 The long gradient times to obtain diagnostic-quality imaging remain a challenge in widespread adoption of IC-VWI and can be especially problematic in clinically unstable patients such as those with acute stroke who may be unable to remain still through the acquisition.
In recent years, there has been increasing interest in the use of artificial intelligence (AI)-based techniques to enhance image quality in IC-VWI. Several of these have used unsupervised or self-supervised networks to reduce the image noise or improve the SNR for T1 SPACE, proton-density, or MRA images.9⇓–11 However, these techniques largely rely on retrospective optimization of prospectively collected data and may not work well in motion-degraded data. More recently, several MRI vendors have introduced both 2D and 3D deep learning (DL)–based image acceleration techniques, which can simultaneously improve image quality and reduce scan times.12,13 Even though these have shown promise in routine clinical practice, there is scarce literature regarding their utility and benefits in IC-VWI. This issue is relevant because these DL-based optimizations are often trained on a broader data set covering different image contrasts and body regions and not specifically on IC-VWI data. The purpose of the current study was to perform a preliminary assessment of the image quality of the optimized deep learning–based image reconstruction (DLBIR) IC-VWI sequences compared with the routine, non-DL accelerated T1-SPACE VWI used in clinical practice, both on pre- and postcontrast images. Herein, we present our preliminary experience with DLBIR for IC-VWI using CAIPIRINHA-based T1 SPACE in healthy volunteers and patients.
MATERIALS AND METHODS
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Image Acquisition
The study was approved by the institutional review board. We acquired noncontrast T1-SPACE IC-VWI using the clinical and DL-optimized sequences in 10 healthy volunteers to compare image quality on the non-contrast-enhanced images. The inclusion criteria were the absence of any current neurologic symptoms such as headaches or any prior brain or spine surgery, no history of diabetes or hypertension, and the absence of any contraindication to the MRI study (for example, noncompatible pacemakers or claustrophobia). We additionally acquired postcontrast T1-SPACE IC-VWI using the clinical and DL-optimized sequences in 5 consecutive patients to compare image quality on contrast-enhanced images. Because these were patients who were getting a clinically indicated study, there were no specific inclusion criteria. Exclusion criteria were the presence of motion (for both patients and volunteers) and the inability to receive gadolinium-based contrast (for patients). However, none of the patients or volunteers were excluded due to the prespecified exclusion criteria. Indications for IC-VWI in these patients included suspected vasculopathy (n = 3), headache (n = 1), and aneurysm (n = 1). All images were acquired on a clinical 3T magnet (Magnetom Vida; Siemens) using a 64-channel head/neck coil. The scanner software version was syngo MR XA50 (Siemens).
The baseline sequence consisted of conventional CAIPIRINHA k-space sampling with parallel imaging reconstruction. The DLBIR sequence was also based on conventional CAIPIRINHA k-space sampling but used DL-based image reconstruction that generates images from k-space data using an architecture inspired by unrolled variational networks.14 By means of undersampled k-space data and precalculated coil sensitivity maps as input, images are formed through 6 iterations comprising a data consistency update and a neural network–based image regularization. The model was trained in a supervised manner using about 5000 training pairs derived from about 500 fully-sampled 3D data sets of healthy volunteers acquired on 1.5 and 3T scanners (Magnetom scanners; Siemens) in the head, abdomen, and pelvis. The obtained model parameters were then exported for prospective use in the scanner reconstruction. With the provided image enhancement, the reconstruction also allows higher acceleration factors for conventional parallel image acquisitions. The same implementation was previously explored for abdominal T1-weighted imaging.15
The scanner parameters for both sequences are provided in Table 1. These parameters were largely similar, other than the use of higher acceleration and DL-based reconstruction in the DLBIR sequence. The scan times for the clinical and DLBIR sequences were 7:26 minutes and 5:23 minutes, respectively.
Technical parameters of the DLBIR and clinical IC-VWI sequences
Image Evaluation
The image quality was independently evaluated by 4 neuroradiologists, each with at least 6 years of neuroradiology experience (range, 6−15 years). All readers were board-certified in radiology and held a Certificate of Added Qualification in neuroradiology. All studies were reviewed on a clinical PACS with multiplanar capabilities. The DICOM headers for the images were hidden to avoid any potential identification of the use of DLBIR technique, with only the series number available to assess and grade image quality. Images from the same subject were evaluated concurrently. Image windowing and use of multiplanar reconstruction were at the discretion of the individual readers.
Image quality was evaluated on a Likert-like scale using a 4-point rating and performed separately for the vessel wall (1 = well defined; 2 = mostly well-defined with some areas of suboptimal visualization; 3 = mostly poorly defined with some well-defined areas; 4 = nondiagnostic) as well as the vessel lumen (1 = uniform flow suppression; 2 = minor artifacts but diagnostic; 3 = prominent intraluminal signal nonsuppression; 4 = nondiagnostic).16 Additionally, the overall image quality was also reviewed in terms of the lowest and highest background noise and image sharpness and homogeneity of CSF signal. A total of 11 vessel segments were evaluated for each sequence (bilateral ICA, MCA, and anterior cerebral artery [ACA], collectively referred to as anterior circulation and bilateral vertebral arteries, posterior cerebral arteries [PCAs], and basilar artery, collectively referred to as posterior circulation). For the MCA, ACA and PCA vessels, image quality was considered until the junction of second- and third-order branches. In patients undergoing IC-VWI with both sequences (n = 5), the depiction of the underlying pathology was also subjectively evaluated in terms of visibility and reader confidence.
Statistical Evaluation
Mean reader scores were evaluated for each vascular segment. Individual segment scores for the vessel wall and lumen were separately calculated for the noncontrast and contrast-enhanced sequences and compared using paired samples t tests. A P value of ≤ .05 was considered significant. Descriptive statistics were used for background noise, image sharpness, and homogeneity of the CSF signal.
RESULTS
The mean vessel wall and lumen visualization scores for the noncontrast images in healthy volunteers (n = 10) are presented in Tables 2 and 3, respectively. The DLBIR images showed overall statistically significant improvement in vessel wall (10/11) and lumen (11/11) depiction in nearly all evaluated segments (Fig 1). For the basilar artery wall visualization, the DLBIR images were rated better in quality though the difference was not statistically significant.
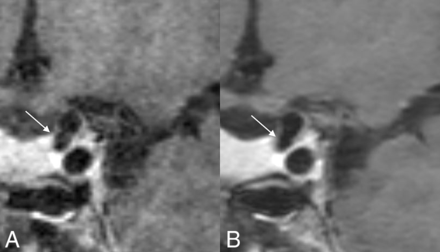
Coronal MPR images of the clinical (A) and DLBIR (B) sequence showing better visualization of the ICA terminus bilaterally, along with reduced background image noise (B).
Mean vessel wall reader evaluation scores for the noncontrast and postcontrast sequences for the IC-VWI
Mean vessel lumen reader evaluation scores for the noncontrast and postcontrast sequences for the IC-VWI
Similar trends were also noted for the postcontrast sequences in patients (n = 5), in which DLBIR images were consistently rated better for wall and lumen visualization (Tables 2 and 3), with the image-quality differences being statistically significant in most segments (vessel wall [7/11]; lumen [7/11]). DLBIR images were consistently rated as having the lowest image noise (60/60 [15 subjects × 4 readers]) and more uniform CSF signal (60/60) by all readers, and as having higher image sharpness (45/60) by most readers. The depiction of various intracranial findings was also consistently noted to be similar or better on the DLBIR images (Figs 2–5). Flow artifacts in the distal vessels were noted to be overall similar but better seen on the DLBIR images, given the reduced image noise and improved image sharpness.
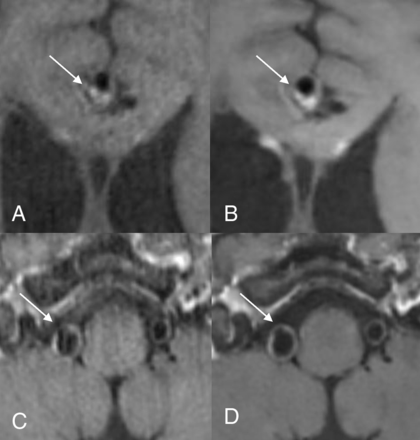
Coronal MPR postcontrast images of the clinical (A) and DLBIR (B) sequence show a medially projecting left ICA aneurysm (arrows). Note that the aneurysm wall is better depicted in the DLBIR image (B).
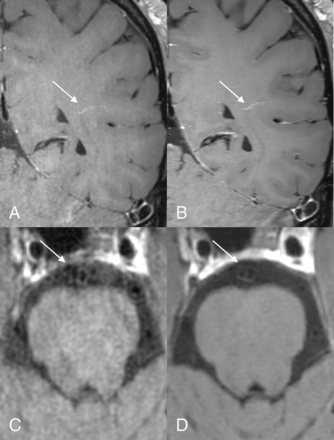
Postcontrast reformatted images in 2 different patients. The upper row shows atherosclerotic involvement in the right A2 ACA segment on the clinical (A) and DLBIR (B) images (arrows). The lower panel, from another patient, shows early atherosclerotic changes in the right V4 segment (arrows). The hypointense wall signal corresponded to underlying calcification on the CT images (not shown) and is better appreciated on the DLBIR image (B).
Coronal MPR postcontrast images in a patient with biopsy-proven cerebral amyloid angiopathy–related inflammation. The vessel wall and surrounding leptomeningeal enhancement (arrows) is appreciated on both clinical (A) and DLBIR (B) images. Note the improved background noise on the DLBIR images.
Upper row images (A and B) from a patient show an incidental developmental venous anomaly in the left temporal region (arrow), equally well seen on the clinical (A) and DLBIR (B) images. Lower row panel (C and D) from another patient with basilar artery fenestration. Image acceleration artifacts in the clinical image (C) almost obscure the underlying fenestration (arrows), which is better seen on the DLBIR images (D).
DISCUSSION
We evaluated DLBIR-based and clinical IC-VWI T1 SPACE sequences for image quality across a subset of healthy volunteers and patients and noted that DLBIR images provided consistently improved vessel wall visualization, reduced intraluminal artifacts, reduced image noise, and uniform CSF signal compared with the clinical VWI sequence, in addition to the approximately 30% reduction in scan times (7:26 (min:sec) for the clinical sequence versus 5:23 (min:sec) for the DLBIR sequence). The depiction of intracranial pathologies was also noted to be similar or better on the DLBIR images. Our preliminary findings are promising in terms of achieving clinically acceptable scan times for IC-VWI and the need to be further validated on a larger cohort.
Given the technically challenging nature of IC-VWI, simultaneously overcoming multiple challenging requirements of improved wall signal, reduced intraluminal flow artifacts, and overall better image quality in clinically reasonable scan times are critical before widespread clinical adoption. In recent years, a few studies have shown that deep neural network–based image denoising methods may outperform conventional image denoising algorithms. This finding has further led to both self-supervised and unsupervised networks being proposed to reduce image noise and improve image quality.9,11,17 Jung et al,11 for example, recently proposed a MR-self Noise2Noise framework to improve the image quality of MPRAGE images on brain scans. The proposed network led to improvements in image quality over conventionally-acquired images. Limited evaluation of T1 SPACE VWI also showed improvement in image quality, though no evaluation of postcontrast images was performed. Additionally, as the previously acquired images were retrospectively denoised, no improvements in scan times were investigated.
Similarly, Zhou et al17 also developed a convolutional neural network to retrospectively enhance acquired turbo spin-echo images with point spread function blurring, demonstrating that images acquired with a longer echo-train length could be retrospectively enhanced to produce image sharpness similar to that of images acquired using a shorter echo-train length. Although the model offers improved image quality and potential scan time savings of about 25% when acquiring sequences with longer echo-train lengths, the convolutional neural network must be retrained for different sequence parameters, especially when changing the echo-train length, TR, and the driven equilibrium condition. Additionally, the impact of convolutional neural network–based processing on postcontrast sequences was not evaluated.
The current work shows that DLBIR images may not only help improve image quality but can provide considerable time savings, allowing easier and widespread implementation in routine clinical practice. The reduced image noise and consequently improved image quality can potentially improve reader confidence in image interpretation. The savings in gradient times provide further room for adding additional tissue-weightings such as T2WI TOF-MRA, while maintaining overall scan times. Even though we evaluated only a limited number of patients with intracranial lesions, these cases included a diverse array of pathologies, including atherosclerotic disease, cerebral amyloid angiopathy–related inflammation, aneurysm, basilar fenestration, and developmental venous anomaly. In all cases, lesion depiction was similar or better on the DLBIR images. For example, the DLBIR images better depicted the aneurysm wall despite proximity to the cavernous sinus (Fig 2). Similarly, visualization of atherosclerotic disease in the distal ACA was again better with the DLBIR sequence, as was the wall calcification in the V4 segment of the vertebral artery (Fig 3). Finally, the basilar artery fenestration in another patient (Fig 5) could be visualized on the DLBIR images, despite near-complete obscuration on the T1 SPACE images secondary to image noise. In the patient who eventually went on to have a brain biopsy and was shown to have cerebral amyloid angiopathy–related inflammation (Fig 4), the DLBIR images had better image quality despite visualization of the pathology on both sequences.
The image-quality rating on various parameters was consistently improved with the DLBIR images. This was mostly statistically significant. Some of the nonsignificant improvements may be secondary to the small sample size or type II error. More importantly, all image-quality parameters trended toward improved ratings (Tables 2 and 3), despite scan time reduction by approximately 30%. Unlike compressed sensing and conventional parallel imaging–based VWI techniques in which reduced scan times are accompanied by a reduction in the contrast-to-noise ratio and image quality, the overall image quality was improved along with reduced image noise, despite a similar reduction in gradient times. This improvement is essentially achieved by a DL-based image reconstruction that is optimized for the same acquisition as used for conventional parallel imaging. Instead of a linear reconstruction used in parallel imaging, the DLBIR sequence uses a k-space-to-image reconstruction based on DL.
Prominent limitations of the DLBIR-based IC-VWI are the vendor-specific nature and limited availability of the sequence currently. However, given the several technical challenges in IC-VWI, these preliminary results are encouraging. Future studies could further explore the usefulness of DLBIR-based IC-VWI in specific patient cohorts such as those with intracranial atherosclerosis, aneurysms, or CNS vasculitis. Future work could also focus on combining DLBIR-based optimization with flow-suppression techniques or compressed sensing–based IC-VWI to explore further optimizations in IC-VWI.
Despite the promising findings, our study has several limitations. We evaluated only a limited number of subjects, given the preliminary nature of the study. The generalizability of these findings would need to be further evaluated in larger cohorts with different patient groups and disease prevalence. The evaluated DLBIR sequences are vendor-specific, limiting generalizability. Other vendor-specific algorithms were not evaluated. Concurrent use of intraluminal flow artifact suppression using the delay alternating with nutation for tailored excitation (DANTE) pulse was not evaluated and may be further assessed in future work.18 Our evaluation was limited to T1-weighted images, and proton-density–based IC-VWI sequences were not evaluated, primarily because most sites currently use T1-weighted sequences for clinical IC-VWI. Some authors have previously shown improved image quality with DL-based denoising on proton-density images as well.9 This result, however, was not the focus of our current work. Finally, objective calculation of the SNR on DLBIR images is mathematically complex, and only Likert-like ratings from multiple readers were considered in the current work.
CONCLUSIONS
Despite the multiple technical challenges in IC-VWI, there is continued enthusiasm and increasing clinical adoption, given its role in cerebrovascular pathologies. The adoption and further optimization of DLBIR methods may improve image quality while simultaneously reducing scan times to clinically acceptable gradient times. Our encouraging preliminary results should be further validated in a larger, multivendor setting.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received April 17, 2024.
- Accepted after revision June 6, 2024.
- © 2024 by American Journal of Neuroradiology