Abstract
BACKGROUND AND PURPOSE: Flow diverters with surface modifications or coatings have been recently introduced to clinical practice with the expectation that they can reduce the rate of thromboembolic complications and residual aneurysms. The purpose of this study is to evaluate the utility of the Derivo 2Heal (D2H) device, a new fibrin and heparin-coated flow diverter.
MATERIALS AND METHODS: Patients treated by a single operator by using the D2H were retrospectively evaluated for demographic data, aneurysm characteristics, procedural variables, and follow-up data. All patients were treated by using a single D2H, monitored by platelet function testing and kept under single antiplatelet therapy with regular or half-dose clopidogrel or prasugrel after the procedure.
RESULTS: Twenty patients with 26 aneurysms were treated. Three presented acutely with subarachnoid hemorrhage. Adjunctive devices were used in 6 patients. There were no technical failures and 2 periprocedural self-limited nonthrombotic minor adverse events. During follow-up, 1 of the acutely ruptured aneurysms reruptured, and 1 patient had a visual TIA. All patients were doing well clinically (19 with mRS of 0 and 1 with 1) at the last follow-up after discharge. The rates of total occlusion on very early angiographic (MRA/CTA or DSA, mean: 2.4 months), DSA (mean: 5.8 months), and midterm angiographic (mean: 14.5 months) follow-up for all versus uncoiled aneurysms were 68% versus 70%, 77.8% versus 90.0%, and 91.7% versus 90.1%, respectively.
CONCLUSIONS: The absence of permanent neurologic deficits in the periprocedural period and favorable occlusion rates in this preliminary study suggest that the novel coating comprising fibrin and heparin may have the potential to increase the safety and efficacy of flow diversion and needs to be further studied by comparing the D2H device with its bare counterpart and other coated or surface-modified flow diverters.
ABBREVIATIONS:
- D2H
- Derivo 2Heal device
- FD
- flow diverter
- HPC
- Hydrophilic Polymer Coating
- RROC
- Raymond-Roy Occlusion Classification
- SAPT
- single antiplatelet therapy
SUMMARY
PREVIOUS LITERATURE:
Surface modification or coating of flow diverters with biopolymers emerged as a promising strategy to reduce periprocedural and delayed thromboembolic complications by lowering surface thrombogenicity of these devices and to potentially improve the rate of aneurysm occlusion. The Derivo 2Heal (D2H) device is the surface treated version of the relatively new Derivo2 device, manufactured by coating of the device with fibrin and covalently binding fibrin onto this coating. There are no current data regarding the clinical and angiographic outcomes related to the use of the surface coated/modified D2H in the current literature.
KEY FINDINGS:
The D2H device demonstrated promising clinical outcomes, achieving comparably high aneurysm obliteration rates with acceptable complication rates under single antiplatelet therapy as compared with the results obtained with the other surface coated/modified devices in current clinical use.
KNOWLEDGE ADVANCEMENT:
The surface modified/coated D2H device holds potential for improving clinical outcomes in aneurysm treatment, warranting further comparative research with bare devices and/or surface treated devices to assess potential early clinical and long-term angiographic benefits.
Since their introduction to endovascular practice approximately 15 years ago, flow diverters (FDs) have evolved to attain higher aneurysm obliteration rates and lower rates of adverse events.1 Even so, there is still a subset of patients in whom FDs fail to obliterate the aneurysm, and there is a nonnegligible rate of—mainly thromboembolic—adverse events.2,3 One of the strategies for improving the safety and efficacy of FDs is the coating of devices with biopolymers in the form of a chemically bound surface modification or physically applied “coating.”4 Currently, the Pipeline Shield (Medtronic), a cobalt-chromium device with a phosphorylcholine-based surface modification and the nitinol-based p48/p64 hydrophilic polymer coating (HPC) devices (phenox) with a glycan-based multilayer polymer coating are 2 such devices in wide clinical use. The FRED X flow diverter (Microvention), which utilizes a poly-2-methoxyethylacrylate polymer surface modification is also currently in clinical use in some countries.5 Balt claims to have developed a coating based on P8RI, a biomimetic peptide of CD31. We report the initial clinical results of using the Derivo 2Heal (D2H) flow diverter (Acandis), which is the first dual surface treated (coated with fibrin and surface modified with heparin) cerebral flow diverter.
MATERIALS AND METHODS
This retrospective study was performed per the Declaration of Helsinki and approved by the local ethics committee. All patients signed an informed consent form before the procedure.
All of the cerebral aneurysms treated in a single institution by using the D2H FD were retrospectively identified. The hospital electronic records of the patients were reviewed for demographic data, aneurysm characteristics (size, shape, location, previous treatments, size of the parent artery), procedural variables (technical success, size of the device, antiplatelet regimen, use of adjunctive devices, technical and clinical adverse events), and follow-up data. Clinical outcome was assessed per the mRS, while the Raymond-Roy Occlusion Classification (RROC) was used to determine angiographic results.
Device Description
The D2H is an FD with 48–52 nitinol composite wires containing a platinum core. The closed distal ends are flared. Similar to the prior version of the device, it has an electropolished (BlueXide) surface that is coated with HEAL technology, which is based on a fibrin mesh with covalently bound heparin.
General Description of the Treatment Protocol
The procedures were performed via common femoral artery access with a triaxial catheter system under general anesthesia and systemic heparin administration. All patients underwent flat-panel CT of the head, and anticoagulation was reversed at the end of the procedure. Femoral puncture site hemostasis was maintained with femoral closure devices.
All patients, except for the 3 patients presenting with SAH, were treated with 75 mg clopidogrel and 300 mg aspirin per day for 5 days before the intervention. The response to clopidogrel was verified by using a point-of-care test. In the case of hyporesponsiveness, clopidogrel was switched to 10 mg/day prasugrel and the patients were tested for platelet responsiveness again. The final level of platelet, obtained before the procedure, is given in Online Supplemental Data. After the implantation of a D2H, all patients received single antiplatelet therapy (SAPT). In the initial phase when we started using the device, 10 mg prasugrel was the standard SAPT, with the only exception being hyperresponsiveness or lack of insurance coverage of the chosen medication. In these 2 latter conditions, 75 mg of clopidogrel was administered instead of prasugrel after the procedure. In the second phase of D2H use, clopidogrel was used as the primary SAPT after the procedure, and prasugrel was only administered if the patient was a clopidogrel nonresponder. In rare cases of clopidogrel hyperresponse, the clopidogrel dose was reduced to 37.5 mg/day as previously reported in the literature.6
Three patients presenting with acute SAH underwent treatment within 24–72 hours after admission. Tirofiban was administered intraoperatively. After the procedure, the patients were gradually switched to oral SAPT. Two patients received a loading dose of clopidogrel and were followed under the same medication whereas the last one was loaded with prasugrel as the patient was treated with telescoping FDs, increasing the metal load in the acute phase of SAH. Because she was found to be a hyperresponder, the medication was stopped for 2 days on postoperative day 1, and the patient was switched to clopidogrel. At 1 week she was still a hyperresponder, the drug dose was reduced to one-half, and she was kept on 37.5 mg as monotherapy. Point-of-care testing after loading showed satisfactory platelet inhibition in the other 2 cases.
After discharge, the patients were called back for a very early clinical and imaging follow-up at 6 to 12 weeks, followed by a DSA follow-up at approximately 6 months. Further follow-up was obtained by noninvasive angiographic imaging. Thienopyridines were switched to 300 mg/day of aspirin after the 6-month follow-up angiogram.
RESULTS
Twenty-six aneurysms in 20 patients were treated by a single operator, and there were 16 female and 4 male patients (average age: 53.1 ± 14.5 years). Two patients had bilateral aneurysms, and 3 had multiple aneurysms on the same arterial segment (2 patients with 2 aneurysms each and 1 with 3 aneurysms). Three patients who were referred from outside institutions were treated within 1 week of subarachnoid hemorrhage and within 24–72 hours after admission. Additionally, 1 patient had a remote history of diffuse subarachnoid hemorrhage. Four aneurysms were in the posterior circulation, 1 was a fusiform middle cerebral artery aneurysm, and the remaining aneurysms were ICA aneurysms. Aneurysm sizes were noted as the maximum diameter, and the average size was 8.0 ± 7.1 mm (range 2–30 mm). None of the patients in this study had any neurologic deficits before the procedure. The demographic data of the patients are listed in the Online Supplemental Data.
All patients were maintained after the procedure on a single antiplatelet drug. Eight were kept on a daily dose of prasugrel 10 mg, and 10 patients were administered 75 mg per day of clopidogrel. Two patients with hyperresponse to clopidogrel were kept on 37.5 mg (half-dose) clopidogrel per day. The procedural details are listed in the Online Supplemental Data.
There were no technical failures. In one patient, bare coiling of the aneurysm with bailout stent placement was planned. During the placement of the initial coil, the coil was unraveled, and further microcatheter maneuvers resulted in microcatheter back-out and partial protrusion of the coil in the parent artery without the ability to release the coil. As a bailout procedure, placement of a D2H across the aneurysm neck with further pinning of the coil to enable coil release was planned. However, the device did not open fully at the level of the protruded coil loop (Online Supplemental Data) despite multiple attempts. After the device was released completely, it showed an hourglass configuration, and it could be bypassed over the delivery wire and apposed properly by using a stentriever device. Otherwise, there were no technical or clinical adverse events during the interventions. In addition to this patient, adjunctive devices were used in 5 other patients. In 4 patients adjunctive coils were used: 2 of these were giant vertebrobasilar aneurysms, one was an aneurysm at the origin of a fetal-type posterior communicating artery, and one was an ophthalmic aneurysm for which the patient specifically asked for coiling in addition to FD placement. In the last patient, telescopic FD placement was planned to treat an acutely ruptured blisterlike ICA aneurysm. Due to a lack of proper sizes in the inventory, a bare (uncoated) Derivo 2 device was placed first, followed by placement of a coated D2H device telescopically inside the bare device.
One patient developed a groin hematoma on the day of the procedure, resulting in a 4.3 mg/dL decrease in the postprocedure hemoglobin level compared with the preprocedure value. The patient was managed medically without endovascular or surgical interventions and was discharged with only tenderness to touch over the puncture site. Another patient with an acutely ruptured, partially thrombosed giant V4 segment aneurysm was treated with coiling of the aneurysm and placement of a coated device. Parent artery occlusion was not performed in this patient as the anterior spinal artery appeared to come off from the aneurysm and there was no obvious anterior spinal branch originating from the contralateral V4 segment or the posterior inferior cerebellar artery. This patient had a second leak from the aneurysm 6 weeks after the procedure and was treated elsewhere with parent artery occlusion. The patient’s mRS score initially worsened, but she recovered within days to baseline neurologic status. Apart from this patient, there was no change in the mRS scores of any of the patients; that is, all of the remaining patients were scored mRS 0 before and after the procedure. A patient treated for an ophthalmic artery aneurysm had an episode of monocular visual loss of the ipsilateral eye lasting for approximately 15 minutes after she was switched to aspirin monotherapy. This patient began clopidogrel monotherapy and has since remained asymptomatic. There were no further clinically relevant events in this cohort.
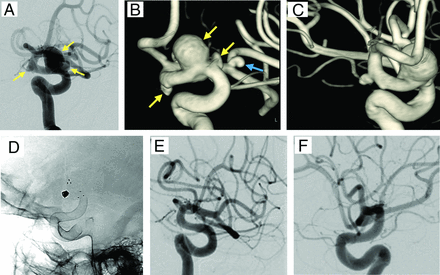
The last patient (Online Supplemental Data) of this cohort had a basilar tip aneurysm that was treated with a variation of “V” stent placement and coiling by using a D2H device and a braided stent (Accero, Acandis). This patient was doing well 2 months after the procedure when a follow-up DSA revealed occlusion of the aneurysm and patency of the stent construct. Because the treatment strategy of this aneurysm was beyond the classic description of flow diversion (total coverage of aneurysm orifice from the proximal healthy artery to the distal healthy artery), the endovascular procedure was included in the safety analysis of the cohort but not in the efficacy analysis. The total (RROC I) overall very early aneurysm occlusion rate at a mean of 2.4 ± 1.6 months (DSA for 10 aneurysms, MRA for 14 aneurysms, and CTA for 1 aneurysm) was 68%, and the rate was similar (70%) when coiled aneurysms were excluded. Early DSA follow-up available for 24 of the 25 aneurysms (DSA was pending for 1 aneurysm in addition to the basilar tip aneurysm that was already excluded from the evaluation of device efficacy) at a mean of 5.8 ± 2.1 months revealed a total aneurysm occlusion rate of 77.8%. When the patient with rerupture (who did not meet the early-term efficacy end point because of parent artery occlusion at 1.5 months) was also excluded in addition to the patient with the basilar tip aneurysm, the mean DSA follow-up was 6.6 ± 2.5 months, and the RROC I occlusion rate increased to 91.3%. The rate was 90.0% when coiled aneurysms were excluded. With these 2 aneurysms (basilar tip and reruptured V4) again excluded for the reason stated above, the final imaging follow-up (2 with DSAs, 4 with CTAs, and 18 with MRAs) at a mean of 14.5 ± 4.6 months showed an overall RROC I occlusion of 91.7% and a total occlusion rate of 90.1% for uncoiled (FD-only) aneurysms. Follow-up imaging results are displayed in the Online Supplemental Data. One patient developed angiographically notable but asymptomatic in-stent stenosis (Online Supplemental Data). This patient was kept on clopidogrel only after the DSA follow-up. The preoperative and follow-up images of patient number 13 are provided as an example in Fig 1.
The internal carotid arteriogram of one of our patients (A) shows 3 tandem aneurysms (yellow arrows). 3D images obtained from the rotational angiogram demonstrate that the anterior choroidal artery originates from the base of the choroidal segment aneurysm (B and C). An incidental middle cerebral artery aneurysm is also noted (blue arrow). A follow-up internal carotid angiogram was obtained 7 months after the initial treatment; the native (D) image and the subtracted (E and F) images in contralateral oblique and lateral views reveal the device and no evidence of residual aneurysms.
DISCUSSION
The safety and efficacy rates of FDs have stabilized over the last several years. The alterations in the physical properties (material, braid number, layer, strut thickness, pore attenuation, etc) within generations of the same device or across different devices increased the technical success rate without a major change3 in the rate of residual aneurysms or complications. Coating the metallic surface of FDs with biopolymers is a promising method to decrease complications, residual aneurysms, and costs/risks associated with dual antiplatelet therapy. The dual, fibrin, and heparin-based HEAL technology of the D2H device was shown to be on a par with the noncoated bare Derivo device in in vitro7 and in vivo8 studies.
The HEAL coating intends to mimic the last step of the natural hemostatic process. A fibrin network is formed in which heparin molecules are subsequently covalently embedded. In its natural form, fully polymerized fibrin is no longer thrombogenic; in contrast, as it “passivates” the surface of the implant, it has antithrombogenic and anti-inflammatory effects.9⇓-11 Endogenous fibrin plays a crucial role in various processes, such as wound healing, in which it serves as a temporary matrix.12⇓-14 The 3D structure of this matrix forms a stable biologic scaffold that allows adhesion and migration of the host cells, resulting in a premature endothelium.13,15⇓-17 As in the other commercially available coated devices, the device coating in D2H aims to provide a rapid restoration of the endothelium with a proportional decrease in the risk of thrombosis and endothelial hyperplasia.3,18
In an attempt to enhance the antithrombogenic properties of the fibrin network, heparin is embedded in the fibrin coating.7,19 Heparin is covalently bound to fibrin at several sites in the molecule. These multiple covalent bonds ensure that heparin acts locally and is not released into the circulation. The coating is therefore noneluting and is not expected to have a pharmacologic effect. Apart from its antithrombogenic effect, heparin, as a negatively charged molecule, repels blood components with the same charge, such as platelets, and is known to enhance endothelialization.19⇓⇓⇓-23 In in vitro experiments, it has already been established that fibrin and heparin achieve a synergistic effect regarding both antithrombogenicity and endothelialization.11 Whether these properties will result in a clinically important benefit remains to be shown.
The in vitro and in vivo data have shown that the combination of fibrin and heparin in a single coating is promising.7,8 However, these data do not necessarily translate into clinical benefit. In this regard, the results of our initial clinical study on D2H FDs are encouraging. Although we used longer devices (for reasons such as extending the proximal and distal landing zones in straight arterial segments, extension of the flow diversion to cover tandem aneurysms with a single device, and sometimes constraints related to the device sizes available in our inventory for any type of device), the 30-day thromboembolic rate and delayed thromboembolic rate was 0% and 5%, respectively, with no permanent events. We showed that under single antiplatelet therapy, in selected cases, under low-dose single antiplatelet therapy, the D2H device was associated with an approximately 90% aneurysm obliteration rate at the 6-month follow-up without permanent morbidity or mortality or technical failure. This occlusion rate is slightly higher than that of the Derivo and Derivo 2 devices without HEAL technology, which have total occlusion rates between 62% and 85%.24⇓-26 The Premier Study27 reports long-term follow-up in a cohort with a slightly smaller size than this study (5 versus 8 mm) yet deserves mention as it reports a longer follow-up. At a rate of 83.3%, the aneurysm occlusion at 3-year follow-up was only modestly lower than the current study. The D2H flow diverter in this cohort also compares favorably with the Pipeline Shield device, which is associated with occlusion rates between 74% and 78%.28,29 Furthermore, the outcomes of single-center self-adjudicated studies with similar patient characteristics (patient number, number of ruptured cases, rate of adjunct device use),28 series with core-laboratory evaluations,30 and a meta-analysis29 analyzing cohorts treated with the Pipeline Shield device are also comparable regarding the perioperative and follow-up data. Results in our patients also compare well with those of the HPC-coated FDs (phenox) in terms of clinical outcome.31⇓-33 There appears to be a higher rate of early-term occlusion in our series than in these latter reports, in which the occlusion rate ranges from 57% under dual anti-platelet therapy (DAPT)32 to 65%–80% under SAPT.31,33,34 The validity or clinical importance of this finding has yet to be proved. In our opinion, based on the outcome reported in this series, undertaking a head-to-head comparison study with the bare device or other coated/surface-modified devices under SAPT is warranted. Such a study will need to address the claims of thrombogenicity and increased aneurysm occlusion rates, which have remained hypothetical as of today, and it is unknown to what extent the biologic modifications of the FDs will be useful clinically.35,36 A recent meta-analysis by Li et al37 that compared the bare, uncoated Derivo 2 device with the Pipeline Shield device revealed no difference in the efficacy or safety of these devices, implying that comparisons between the bare and coated versions of the same device are mandatory to reveal a possible effect of a specific coating. Although such a randomized trial is underway to show this possible difference, the primary end point (DWI results) is not primarily clinical, nor is it a surrogate marker that is specific to the thrombogenicity of the device.38 Hence, further data from individual or multiple centers are needed to identify specific clinical settings or antiplatelet treatment protocols for which randomized comparisons of the coated and bare devices are justified to clarify enhanced safety or efficacy.
The major limitations of this study are the retrospective design, small patient cohort, and self-adjudicated outcomes. Additionally, we were unable to provide long-term DSA follow-up because our routine practice does not involve invasive long-term imaging due to the acceptable diagnostic accuracy of MRA, especially with nitinol devices like the D2H device.39 The utility of MRA instead of DSA can potentially be a reason for the apparent increase in the rate of aneurysm occlusion during follow-up. However, the study provides encouraging data for the evaluation of the coated device with a single type of thienopyridine in a larger prospective series, including evaluation of the results by external, independent facilities. It should be noted that our study does not include any patients treated or followed by aspirin only and our results should not be generalized for patients who will be treated or followed by aspirin monotherapy.
There are several implications of our study. We have shown that the technical handling of the D2H is similar to the uncoated version, with a similar rate of success. The initial outcome was also within the expected range in terms of periprocedural and follow-up clinical findings. Both of these results favor further clinical investigation of this device. As a final point, we demonstrated that reduced dose SAPT with clopidogrel, a relatively less potent drug, may be a possible alternative drug regimen in flow diversion based on antiplatelet testing. It is very important to record that the utility of such a drug regimen may not be restricted to the coated device yet may theoretically be influenced by variables including the physical properties of a specific device and the clinical characteristics of patients.
CONCLUSIONS
The D2H device with its fibrin/heparin coating resulted in favorable clinical and imaging outcomes in this preliminary study. Comparative studies with the bare device are needed to relate any improvements in thromboembolic complication rates and aneurysm occlusion rates to the device coating.
Acknowledgments
A.A. designed the study and performed the interventions. A.A. and A.U. wrote the paper. A.A., A.U., F.C., and S.B. collected and reviewed the data. All authors contributed to the article and approved the submitted version.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received November 20, 2023.
- Accepted after revision March 9, 2024.
- © 2024 by American Journal of Neuroradiology