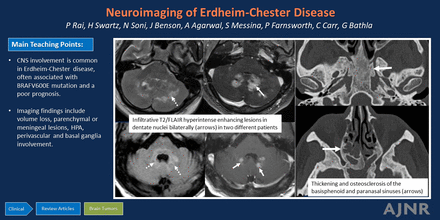
Graphical Abstract
Abstract
SUMMARY: Erdheim-Chester disease (ECD) is a rare, multisystem histiocytic disorder characterized by its variable clinical presentations. CNS involvement is observed in approximately one-half of patients with ECD (up to 76% in some series) and often carries a poorer prognosis. While CNS involvement may remain asymptomatic, others may experience a range of neurologic symptoms, including cognitive decline, neuropsychiatric disturbances, motor deficits, cranial or peripheral neuropathies, and endocrine abnormalities. Neuroimaging findings in CNS-ECD are diverse, including neurodegeneration manifesting as cerebral or cerebellar volume loss; solitary or multifocal variably enhancing intraparenchymal lesions along the neuroaxis; meningeal infiltration; and involvement of the hypothalamo-pituitary axis, perivascular sheathing, or basal ganglia lesions. Other well-documented sites of involvement include the craniofacial region, orbits, and spine. Awareness of these findings is relevant, not only because of the nonspecific nature of these findings, but also because of the high proportion of CNS involvement in ECD and the higher mortality associated with CNS involvement. This review provides an in-depth overview of the various manifestations of CNS involvement in ECD and their imaging features, along with a brief overview of the differential considerations, which include other histiocytic and nonhistiocytic processes.
ABBREVIATIONS:
- CE
- contrast-enhanced
- CLIPPERS
- chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids
- ECD
- Erdheim-Chester disease
- GWP
- granulomatosis with polyangiitis
- HPA
- hypothalamo-pituitary axis
- LCH
- Langerhans cell histiocytosis
- MAPK
- mitogen-activated protein kinase
- MEK
- mitogen-activated protein kinase kinase
- RDD
- Rosai-Dorfman disease
Erdheim-Chester disease (ECD) is a rare non-Langerhans cell histiocytosis, first described in 1930 by 2 pathologists, Jacob Erdheim and William Chester.1 While previously classified as an inflammatory disorder, it was reclassified as a hematopoietic neoplasm according to the 2016 World Health Organization classification. The revised 2016 classification of histiocytosis by the Histiocyte Society proposes 5 subtypes or categories of histiocytosis (L, C, R, M, and H) depending on characteristics that are clinical, radiologic, pathologic, phenotypic, genetic, and molecular. Out of these categories, both ECD and Langerhans cell histiocytosis (LCH) are included in the “L” (Langerhans) group, and Rosai-Dorfman disease (RDD) in the R group.2 Even though CNS involvement in histiocytic disorders can have overlapping imaging manifestations, CNS involvement is uncommon in RDD (<5%) and is predominantly confined to the hypothalamic-pituitary region (92.9%) in LCH.3⇓⇓-6 The current work, therefore, is focused on imaging findings in ECD in the CNS, unless stated otherwise.
The exact prevalence of ECD remains undetermined. However, approximately 1500 cases have been documented in the English literature,7 with patients typically presenting in the fifth decade of life.8 The interval between symptom onset and diagnosis can range from several months to as long as 25 years, often attributed to misdiagnosis due to the absence of a specific clinical syndrome and the rarity of the disease itself.9 Men are more frequently affected than women. The disorder can have a wide spectrum of clinical presentations, ranging from clinically indolent to life-threatening, potentially fatal disease. Patients with ECD have a median survival of 13.5 years and a 5-year survival rate of 82.7%.3
ECD is a multisystemic disorder and can involve virtually any organ system. The long bones of the upper and lower extremities are, however, most commonly affected, manifesting as symmetric medullary sclerosis.10 Though CNS involvement is relatively common with systemic disease, isolated CNS disease is uncommon and primarily reported in case studies.3,11 Notably, CNS involvement is an independent adverse prognostic factor and is associated with higher age at disease onset when compared with those without CNS involvement (median, 62 [range, 23–76] years versus 45 [range, 25–70] years; P = .03).12
This review article explores the common and rare imaging manifestations of ECD within the CNS and discusses notable imaging differentials and cues that may help in accurate diagnosis. Given the diagnostic challenges and protean imaging phenotypes of CNS-ECD, a thorough understanding of imaging findings and imaging mimics may be helpful for timely and accurate diagnosis.
PATHOPHYSIOLOGY AND HISTOPATHOLOGIC FEATURES
ECD is a neoplastic clonal myeloid form of histiocytosis, driven by mutations in the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathways. Mutations affecting the MAPK signaling pathway are present in over 80% of patients with ECD.9 Of these, BRAFV600E mutation is the most common (57%–70%), followed by MAP2K1 mutations (20%).13 The identification of BRAF and other MAPK pathway abnormalities, along with the observed co-occurrence of ECD and LCH in 15% of patients with histiocytosis, were important determinants of the 2016 reclassification of histiocytosis.2
Mutations in the MAPK pathway result in uninhibited proliferation of histiocytes, which are positive for CD68, CD163, Factor XIIIa, and Fascin, negative for CD1a and Langerin/CD207 (which are positively seen in LCH) with a variable expression of S-100.14 Histologically, ECD is characterized by infiltrates of large, lipid-laden histiocytes (xanthoma cells) within a chronic inflammatory background of lymphocytes and plasma cells, with occasional Touton giant cells and fibrosis.15 The identification of BRAF mutations offers the potential for targeted therapy with FDA-approved agents like Vemurafenib.16 In patients with wild-type BRAF, treatment regimens incorporating mitogen-activated protein kinase kinase (MEK) inhibitors such as cobimetinib have demonstrated promising outcomes.17
CLINICAL FINDINGS
ECD presents with a diverse range of clinical manifestations that vary in frequency but can impact both disease diagnosis and prognostication. In many instances, the disease has an indolent course and may be discovered incidentally during evaluation for another condition. The most common symptom is bone pain, as bone involvement occurs in approximately 95% of cases.18 Bone involvement is characterized by bilateral symmetric medullary sclerosis of the meta-diaphysis of long bones in the appendicular skeleton, with less frequent involvement of the skull and axial bones. This pattern of sclerosis contrasts with LCH, where lytic lesions are more common.19 Table 1 summarizes the differences between CNS manifestations of common histiocytic disorders.
Comparison between the CNS manifestations of the common histiocytic disorders
Patients with ECD exhibiting neurologic symptoms also often have concurrent bone lesions. Most patients with CNS-ECD are symptomatic, with asymptomatic involvement only seen in about 11%.3 Neurologic manifestations vary, based on the site and severity of involvement, and can include cognitive impairment, neuropsychiatric disturbances, motor deficits, cerebellar dysfunction, cranial or peripheral neuropathies, seizures, oculomotor abnormalities, and myelopathy. Cognitive difficulties and neuropathies are the most commonly reported symptoms.20 Additional symptoms indirectly associated with CNS involvement include headaches (secondary to meningeal involvement), diabetes insipidus (pituitary involvement), and exophthalmos (orbital involvement).21
A higher prevalence of the BRAFV600E mutation has been observed within the subset of patients with ECD with CNS involvement (60%–77%).3,4,22 A systematic review by Cives et al23 revealed that patients with CNS involvement were less likely to exhibit cardiac involvement, albeit nonsignificantly, and had a higher incidence of bone, skin, retroperitoneal, lung, aortic, and renal infiltration. Furthermore, over 50% of these patients experienced simultaneous involvement of at least 2 distinct anatomic sites within the CNS.23 CNS involvement has also been independently identified as a predictor of both mortality and refractoriness to first-line therapies. Consequently, early detection of ECD in the CNS can potentially reduce the morbidity associated with ineffective immunotherapies and treatments.24
Additional systemic symptoms may also be seen, depending on the site and severity of involvement of the various organ systems. Myeloproliferative disorders and myelodysplastic syndromes are also seen in approximately 10% of ECD cases.25
NEUROIMAGING FEATURES
Neurodegeneration
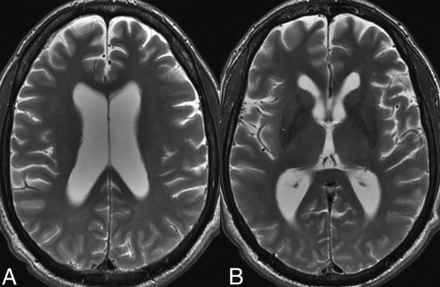
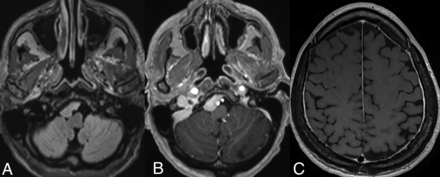
Prevalence of parenchymal volume loss (Fig 1) as a manifestation of underlying ECD has been reported in approximately 15% of patients (over 60% in some studies).3,4,26 Notably, most studies examining this relationship have relied on subjective or ordinal assessments rather than quantitative volumetric measurements. The underlying pathophysiology is hypothesized to be uncontrolled systemic inflammation resulting in elevated levels of proinflammatory cytokines, which can readily traverse the blood-brain barrier. The resulting inflammatory cascade and microglial activation results in neuronal loss and disruption of myelin integrity, ultimately leading to cortical and cerebellar atrophy. The term “pseudodegenerative” has been used by some authors because areas of axonal degeneration and myelin loss may not be accompanied by any histiocytic infiltration.3,27
Parenchymal atrophy. Axial T2-weighted images in a 55-year-old patient with ECD demonstrate diffuse cortical atrophy with mild ex-vacuo dilation of the lateral ventricles. There were no focal lesions.
Diamond et al28 conducted a volumetric analysis in a small cohort (n=11) of patients with ECD without CNS tumors or prior neurotoxic therapies, demonstrating diffuse reductions in cortical thickness and subcortical white matter. Their findings suggest that cognitive decline and behavioral changes in patients with ECD may, in part, be secondary to reduction in the brain volume. Even though there were no statistically significant differences in white matter or cerebellar volumes between patients with ECD and age-matched controls, the former showed a statistically significant reduction in cortical thickness and subcortical gray matter volume. Bhatia et al22 observed disproportionate cerebellar atrophy compared with cerebral hemisphere volume loss in 14% of their study population. Similar findings were observed in the study by Zahergivar et al4 where infratentorial (cerebellar and brainstem) atrophy was observed more commonly than cerebral (20.7% versus 13.8%), indicating that in a subset of patients, there may be differential involvement of the infratentorial brain.4,22 The latter also attempted to establish a correlation between cerebral atrophy and BRAFV600E mutation. While their findings narrowly missed statistical significance (P = .053), the observed trend suggests the need for further research to investigate this potential association.4
Parenchymal volume loss may also be seen with other histiocytic disorders and may be proportionately more common with LCH. Fan et al,26 for example, compared neuroimaging findings between LCH, ECD, and RDD and noted that both LCH and ECD exhibited degenerative patterns, with cortical atrophy being more prevalent in LCH.3,6
Parenchymal Lesions.
These may involve the supra- or infratentorial brain. While initial studies found a predilection of these lesions for the posterior fossa,29,30 Boyd et al,20 in their prospective study, found a broad neurologic involvement throughout the craniospinal axis.
Lesions in the supratentorial compartment (seen in approximately 46% of cases) are encountered more frequently in the frontal lobe, followed by the parietal and occipital lobes (Fig 2). Intraparenchymal enhancing lesions can show considerable variation in size and were classified by Fan et al26 into micronodular (<3 mm), nodular (3–10 mm), and masslike (>10 mm) subtypes. These lesions can be solitary to multiple, sometimes resembling metastatic disease.31 On imaging, they typically exhibit T2 prolongation without any restricted diffusion, often have heterogeneous enhancement, and show minimal associated edema and mass effect.20 On perfusion imaging, the intraparenchymal lesions demonstrate increased Ktrans values, either with or without increased Vp values.22
Parenchymal lesions. T1-CE maximum intensity projection images in a patient (A) reveal scattered micronodular enhancing lesions bilaterally. Axial T2 WI (B) and T1-CE images (C) in a different patient demonstrate a masslike T2 hyperintense periventricular lesion with homogeneous enhancement and mild surrounding edema.
White matter involvement is variably described (60%–87%) and manifests as nondiffusion restricting supra- and infratentorial lesions with or without underlying enhancement. There is a lack of clarity about these lesions, with some authors considering them as a component of the neurodegenerative changes,3,26,32 and others classifying them as parenchymal lesions attributable to ECD,33,34 and the remainder not explicitly labeling these into either subtype.30 Without a histopathologic diagnosis of these lesions, which is generally not performed in all cases, the exact etiology and definitive diagnosis remain uncertain and offer a further scope for research. Given that ECD is more commonly seen in patients in the fifth to seventh decade of life, it is conceivable that at least some of the nonenhancing lesions may overlap with underlying leukoaraiosis. Within the deep gray structures, basal ganglia involvement may occur in about 7% (most commonly putamen), followed by the amygdala and thalamus.4 Less frequently described imaging findings in the basal ganglia involvement include multiple punctate areas of signal loss on SWI in a pattern atypical of senile calcifications (Supplemental Data).35 However, the precise implication of these findings remains unclear and warrants further investigation.
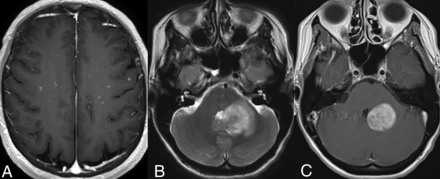
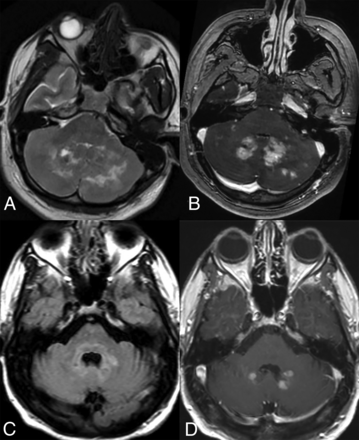
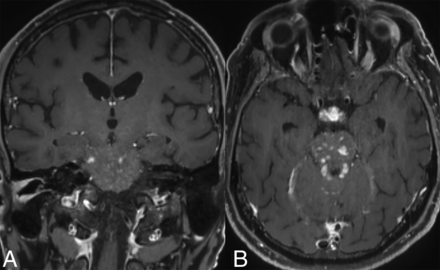
Infratentorial compartment involvement (Fig 3) is present in approximately one-half of all cases, affecting both the brainstem and cerebellum, which may show volume loss and enhancing or nonenhancing lesions. The BRAFV600E mutation is frequently associated with cerebellar involvement, often characterized by the presence of bilateral lesions and frequent involvement of the dentate nucleus (15%).4,15,29 Involvement of the bilateral superior or middle cerebellar peduncles is more common, with the inferior cerebellar peduncles less frequently affected. Within the brainstem, the pons is commonly involved, followed by the medulla and the midbrain.4 The affected structures may also exhibit atrophy over time.34
Cerebellar involvement. Axial T2-WI (A) and T1-CE (B) images in a patient demonstrate ill-defined T2-isointense lesions in bilateral cerebellar hemispheres, most prominently along the dentate nuclei. Axial FLAIR (C) and T1-CE (D) images in a different patient reveal more focal involvement of the bilateral dentate nuclei.
ECD involving the spine can manifest in a uni- or multifocal pattern (Fig 4). Imaging findings may mimic demyelinating or inflammatory lesions.30,34,36 Due to the rarity of spinal involvement, however, there is debate whether routine spine screening is required in the absence of symptoms.22
Spinal involvement. Sagittal T1-CE image (A) reveals tiny discrete enhancing foci involving the cervical cord and the occipital lobe. Sagittal T2 WI (B) and T1-CE images (C) in a different patient demonstrate a masslike involvement of the conus.
Uncommon CNS-ECD manifestations include curvilinear enhancement along the ependymal lining of the lateral ventricle with extension into the lentiform nucleus (Supplemental Data), cystic lesions with septal or ring enhancement, pineal or choroid plexus masses, and diffuse T2 hyperintensity of the pons with or without irregular transversely oriented enhancement.15 Occasionally, punctate-enhancing lesions similar to chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS) may also occur (Fig 5).26
Coronal (A) and axial (B) T1-CE images in a patient with ECD demonstrate punctate areas of enhancement relatively confined to the pons, mimicking CLIPPERS. Note the thickening and enhancement within the infundibulum in (B) suggesting involvement of the HPA axis by the disease.
Intraparenchymal lesions within any part of the neuraxis may exhibit prolonged gadolinium contrast retention, with some demonstrating a T1-shortening effect days after initial imaging.37,38 The exact mechanism underlying this finding remains unclear, but it is hypothesized to be attributed to the abnormal retention of gadolinium by the histiocytes.39
Hypothalamo-Pituitary Axis (HPA) Lesions.
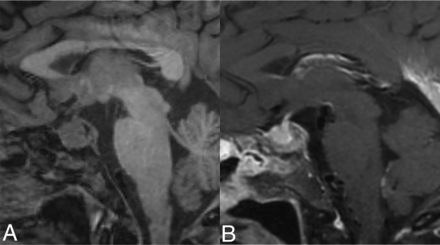
HPA involvement is observed in approximately 17%–44%.3,4,22,30 The most common imaging manifestation is the loss of posterior pituitary bright spot followed by pituitary stalk thickening.40 Other findings include enhancing nodular or micronodular lesions involving the sella or infundibulum (Fig 6), deviation of the stalk, pituitary atrophy, or T2 hyperintensity within the hypothalamus.10,26,30,32
HPA axis involvement. Sagittal T1 noncontrast image (A) demonstrates an enlarged pituitary gland with loss of the posterior pituitary bright spot. Also, note abnormal marrow signal in clivus from bony involvement. Sagittal T1-CE image (B) demonstrates heterogeneous enhancement.
Diabetes insipidus is seen in nearly 50% of patients with CNS-ECD, secondary to HPA involvement.41 Larger lesions may also present with anosmia or features of hypopituitarism. Endocrine disorders and HPA involvement are more common in LCH as compared with ECD.26
Vascular Involvement.
Vascular involvement is seen in approximately 10%–17% of the cases and is unique to ECD compared with LCH and RDD.10,26 When present, it is associated with a poor prognosis and higher mortality as the disease progression ultimately leads to fibrosis and persistent, progressive ischemic manifestations.26,32
Direct vascular involvement in ECD occurs because of periadventitial vessel inflammation, in contrast to the transmural inflammation seen in other vasculitides like Takayasu arteritis. This inflammation can lead to a secondary mass effect and compression of adjacent structures.
The vascular sheathing may exhibit a circumferential or noncircumferential segmental pattern of T2 isointense to hypointense, perivascular soft tissue with homogeneous enhancement (Fig 7), predominantly involving the vessels of the posterior fossa such as the basilar trunk or the vertebral arteries.42 Involvement of these or any of the vessels of the anterior circulation may also result in secondary stroke.43 Vascular involvement in the neck, near the carotid bulb, may present as dysautonomia.20 Vessel wall imaging can be helpful in indeterminate cases.
Vascular involvement. Sagittal (A) and axial (B and C) T1-CE in 3 different patients with ECD showing vascular involvement. There is vertebral artery involvement (short arrow, A) along with dural, tentorial, and cavernous sinus involvement (long arrows, A). In the second patient (B), there is vascular sheathing and enhancement around the right vertebral artery (arrow, B). The third image (C) shows similar involvement of the ICA vessels (arrows) along with orbital infiltrative lesions (black stars).
ECD presenting as dural masses may also secondarily involve the dural venous sinuses (Supplemental Data), with the sagittal, transverse, or straight sinuses being commonly affected, leading to thrombosis.10,44 With pericarotid vascular sheathing, secondary extension into the cavernous sinuses (Supplemental Data) may be observed.10 The presentation may be chronic, and the lesions tend to involve the venous sinuses focally without any intraluminal extension. Secondary venous infarcts, however, have not been reported in the literature.
Extra-Axial Lesions.
Dura-based lesions, seen in approximately 30%–50% of cases,8 generally involve the supratentorial compartment in the form of single or multiple dural-based masses. They are generally T2 hypointense, and invariably enhance on postcontrast sequences.22,40 Similar to the parenchymal lesions, they frequently demonstrate increased Ktrans values on perfusion imaging, either with or without increased Vp values.22 While their imaging characteristics may resemble a meningioma,32 concurrent parenchymal lesions or systemic disease favors the former, while adjacent bony hyperostosis and calcifications within the mass favor the latter. Another reported imaging finding is the presence of radiating T1- and T2-weighted hypointense spicules originating from the center of the mass, which do not exhibit enhancement.15 Solitary fibrous tumors may also exhibit somewhat similar findings, characterized by a “yin-yang” appearance with varying degrees of low and high T2 signal intensity within different areas of the lesion. This can make it challenging to differentiate from extra-axial lesions associated with ECD, especially in the absence of intraparenchymal lesions or systemic disease.45
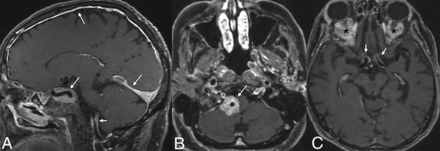
The presence of dural masses makes patients with CNS-ECD susceptible to spontaneous atraumatic subdural hematomas.46 Extra-axial involvement can also manifest as diffuse pachymeningeal thickening (Fig 8) and enhancement over cerebral or cerebellar convexities.47
Axial FLAIR (A) and T1-CE (B) images demonstrate an enhancing dural-based lesion along the clivus. A T1-CE (C) image of another patient reveals diffuse pachymeningeal enhancement.
Spinal extradural involvement with ECD may present with compressive myelopathy (Supplemental Data). Lesions may also mimic schwannomas when involving the exiting nerve roots.48 Patients may occasionally have concurrent paraspinal muscle involvement, presenting as enhancing, infiltrative masses. However, the lack of any pathognomonic imaging findings in ECD makes it challenging to accurately diagnose the condition, especially without a comprehensive clinical context.
Leptomeningeal disease in ECD is uncommon (6%–7%) and may manifest as smooth or nodular leptomeningeal enhancement (Supplemental Data).8,49 The presence of meningeal disease may not necessarily be accompanied by signs of clinical meningitis. CSF analysis generally demonstrates elevation of protein, decrease in glucose levels, and rarely, the presence of foamy histiocytes.49,50
Craniofacial Involvement.
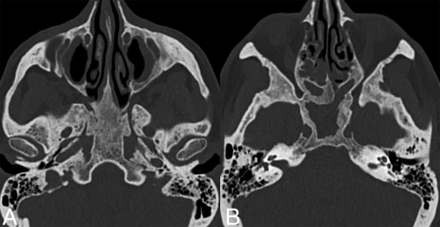
Craniofacial involvement in ECD is seen in 40%–50% of cases and manifests as discrete or confluent osteosclerotic lesions with variable bony thickening (Fig 9). The most frequently affected bone is the calvaria, followed by the skull base and paranasal sinuses. On MRI, they typically appear hypointense on both T1- and T2-weighted images.3,22,40
Sinonasal ECD. Axial CT images (bone kernel) in a patient with ECD demonstrate skull base and sinus involvement characterized by thickening and osteosclerosis of the basisphenoid and paranasal sinuses.
Calvarial involvement in ECD is less frequent than in other histiocytosis (such as LCH). It is frequently accompanied by concurrent involvement of other skeletal sites, such as the ribs and long bones of the appendicular skeleton.30,51 For patients with potentially referable neurologic symptomatology, a targeted CT scan of the sinuses and/or skull base may help find causative osseous lesions.22
Orbital Involvement.
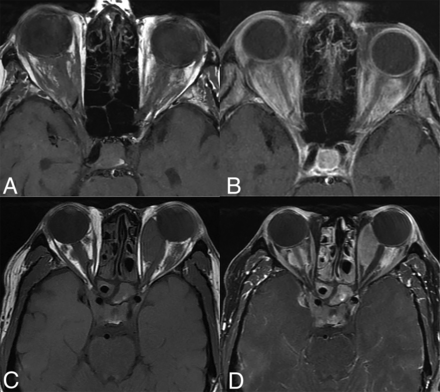
Orbital involvement in ECD is typically bilateral (18%–30%), with the intraconal compartment most commonly involved (Fig 10).3,18,40 Both infiltrative and masslike involvement may occur and present with T2WI hypointensity and enhancement. The resulting mass effect and compression of the optic nerve can lead to proptosis, visual deficits, retro-orbital pain, and oculomotor nerve palsy.20,21 In some cases, the lesions may extend into the extraconal compartment to secondarily involve the lacrimal gland.52 Involvement of the optic chiasm, however, is uncommon.20 It is also notable that orbital involvement is higher than in LCH and RDD, which may help differentiate between these entities.26
Orbital involvement in ECD. Axial T1 WI precontrast (A and C) and T1-CE (B and D) images of 2 different patients showing infiltrative (A and B) and masslike (C and D) orbital involvement. There is involvement of the bilateral cavernous sinuses in both cases along with paranasal sinus involvement in the bottom row (C and D).
Primary optic nerve involvement, characterized by optic nerve sheath enlargement or signal abnormalities, is observed in approximately 47% of cases.53 Choroidal involvement, though rare in ECD, may present as a masslike intraorbital lesion.54 Table 2 summarizes the common neuroimaging manifestations of ECD.
Summary of the CNS imaging findings in Erdheim-Chester disease (reported prevalence in parentheses)
POSTTREATMENT IMAGING FINDINGS IN ECD
A contrast-enhanced (CE) MRI of the brain with gadolinium is recommended for all patients with ECD at the time of diagnosis.21 Regular follow-up imaging is crucial for patients with CNS involvement undergoing treatment, preferably more closely during the early stages.34 Treatment options include surgical resection, radiation therapy, high-dose corticosteroids, targeted agents, and other systemic therapies.22
There is a limited body of literature on the posttreatment imaging features of CNS-ECD. Many patients with CNS involvement undergo debulking surgery for extra-axial masses or receive BRAF/MEK inhibitors due to their rapid response.47 Post treatment, lesions often demonstrate a reduction in size and enhancement (Supplemental Data), though residual deficits may persist in patients with nonenhancing lesions. Surrounding edema may also show interval improvement.15,55 In some cases, near-complete remission of imaging findings may be seen.47 Cohen Aubart et al3 noted that MRI findings are generally concordant with disease activity, and it is rare to have worsening of imaging findings in patients who are clinically improving or stable. In general, however, clinical status is a more reliable indicator of treatment effectiveness.22
DIFFERENTIAL DIAGNOSIS
Given the multiple and often nonspecific imaging manifestations, CNS-ECD can have a broad differential diagnosis. The closest differential considerations are other histiocytosis subtypes (eg, LCH and RDD), and histopathologic examination may be the only definitive means of differentiation. In their comparative study, Fan et al26 noted that CNS-LCH lesions are often solitary and invariably involved the hypothalamic-pituitary region. RDD, on the other hand, may have overlapping manifestations with CNS-ECD but is considerably rarer and involves a younger age group. Vascular involvement was exclusively seen with CNS-ECD. Similarly, bony lesions are rare in RDD, lytic in LCH, and invariably sclerotic in ECD.
Brain metastases may present as enhancing parenchymal lesions but are considerably more common, often cortical in location, and may have associated hemorrhage or cystic change. Vascular sheathing, CLIPPER like pattern, T2 shortening, and concurrent bilateral craniofacial involvement would be atypical. Similarly, hypothalamic-pituitary involvement may occur but is generally uncommon. A whole-body PET-CT scan can aid in identifying the primary tumor site.56 In equivocal cases, a stereotactic biopsy may be warranted. Leptomeningeal carcinomatosis may also mimic CNS-ECD but can be differentiated based on CSF cytology and biochemical findings.57
Patients with primary CNS lymphoma are generally older (unless immunocompromised) and more likely to show diffusion restriction in lesions. Involvement of the HPA axis and dura is less common, as is parenchymal atrophy in untreated patients.58 Neurosarcoidosis, on the other hand, can have several overlapping imaging manifestations in terms of parenchymal, dural, and meningeal involvement. However, the bony craniofacial and orbital involvement is less common and more focal when present. Dilated medullary veins, as noted in a subset of patients with neurosarcoidosis, are not present in CNS-ECD.59,60 Spinal involvement in sarcoidosis generally involves ≥3 vertebral segments.61 Patchy brainstem involvement can also occur in the context of CLIPPERS or intravascular primary CNS lymphomas, but these conditions generally show a dramatic response to steroids, unlike ECD.62
CNS-ECD and granulomatosis with polyangiitis (GWP, previously Wegner granulomatosis) both may show meningeal, sinus, and orbital involvement. However, GWP typically shows osteolytic lesions with nasal septal erosion and frequently shows extraconal and unilateral orbital involvement, while long-bone involvement is rare.63
CNS IgG4-related disease predominantly affects the pituitary gland and meninges rather than the parenchyma and cranial nerves. Hypothalamic-pituitary axis involvement can manifest as thickening or infiltration of the infundibulum or enlargement of the pituitary gland. The most common site of orbital involvement is the lacrimal gland followed by the extraocular muscles or intra- or extraconal orbital fat.64,65 Orbital lesions may resemble pseudotumors on imaging, with definitive diagnosis often requiring biopsy for confirmation.
CONCLUSIONS
While the imaging features of CNS-ECD may overlap with other diseases, a high index of clinical suspicion and a pattern-recognition approach can aid in diagnosis. Given that CNS involvement is noted in most cases, it is essential that neuroradiologists are familiar with the diverse imaging manifestations of this condition. The association of CNS-ECD with a poorer prognosis and a higher incidence of BRAFV600E mutations further underscores the importance of accurate diagnosis and targeted therapy (with BRAF or MEK inhibitors). Awareness of the neuroimaging spectrum of ECD can play a crucial role in early detection and guiding treatment decisions.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.
- 30.
- 31.
- 32.
- 33.
- 34.
- 35.
- 36.
- 37.
- 38.
- 39.
- 40.
- 41.
- 42.
- 43.
- 44.
- 45.
- 46.
- 47.
- 48.
- 49.
- 50.
- 51.
- 52.
- 53.
- 54.
- 55.
- 56.
- 57.
- 58.
- 59.
- 60.
- 61.
- 62.
- 63.
- 64.
- 65.
- Received September 12, 2024.
- Accepted after revision November 7, 2024.
- © 2025 by American Journal of Neuroradiology