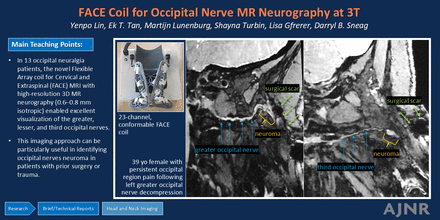
Graphical Abstract
SUMMARY:
This technical report describes use of a novel, conformable receive-only radiofrequency coil for 3T MR neurography in a cohort of patients with occipital neuralgia. Applying a submillimeter, isotropic 3D double-echo steady-state sequence, detailed visualization of the occipital nerves and associated pathologies could be achieved.
ABBREVIATIONS:
- DESS
- double-echo steady-state
- DRG
- dorsal root ganglia
- ETL
- echo train length
- FACE
- flexible array for cervical and extraspinal
- GON
- greater occipital nerve
- HNU
- head-neck unit
- LON
- lesser occipital nerve
- PSIF
- reversed fast imaging in steady-state free precession
- RBW
- receiver bandwidth
- TON
- third occipital nerve
- ZTE
- zero-echo time
Occipital neuralgia may result from pathologic changes of the greater occipital nerve (GON), lesser occipital nerve (LON), and/or third occipital nerve (TON). Targeted interventions, such as analgesic injections or surgical decompression, can provide substantial pain relief.1 However, as clinical symptoms of occipital neuralgia overlap with those of other headache disorders,2 arriving at a definitive diagnosis can be challenging.
MR neurography has emerged as a promising technique for evaluating patients with occipital neuralgia, which may exhibit asymmetric hyperintensity and caliber changes of the GON as it emerges from the semispinalis capitis muscle and trapezius fascia to then enter the overlying subcutaneous fat.3 Additionally, MR neurography can identify neuroma formation after iatrogenic injury.4⇓–6 3D sequences such as TSE/FSE and reversed fast imaging in steady-state free precession (PSIF) are often used in MR neurography to delineate the circuitous courses of the small peripheral nerves in the head and neck.3,7 An alternative 3D double-echo steady-state (DESS) sequence, similar to PSIF, is more time-efficient because it acquires 2 echoes with minimal increase in repetition time, and in theory, can provide the high spatial resolution needed for visualizing the occipital nerves, from the C2–C3 dorsal root ganglia (DRG) to their subcutaneous innervations.8 Further image quality enhancement for 3D DESS sequences have also been demonstrated by using deep learning and image combination reconstructions.9 Even when applying these advanced reconstruction techniques, our experience with the DESS sequence by using a conventional, 21-channel rigid head-neck unit (HNU) is that insufficient SNR is available to acquire submillimeter, isotropic resolution.
The development of flexible coil technologies can improve conformability of surface coils to increase SNR, and enables specialized coil designs that can be optimized to specific anatomic regions.10 Recently, a 23-channel flexible array coil for cervical and extraspinal (FACE) MRI was developed for the posterior and lateral neck regions.11 The FACE coil demonstrated superior SNR and improved parallel imaging performance compared with a conventional 21-channel HNU.11 Its use in patients with occipital neuralgia is expected to improve confidence in imaging the occipital nerves and identifying related pathologies. This work describes the application of the FACE coil, and acquisition and reconstruction algorithms, for MR neurography evaluation of a cohort of patients with occipital neuralgia.
MATERIALS AND METHODS
This prospective study was approved by our institutional review board with written informed consent obtained for utilizing the prototype coil. The study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines. Between November 2022 and February 2024, patients with clinically suspected occipital neuralgia who underwent MR neurography on a 3T MRI system (Signa Premier, GE Healthcare) were enrolled.
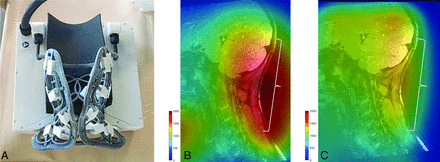
The FACE coil11 (Tesla Dynamic Coils) comprises a flexible mask for optimal neck contouring and a rigid posterior headrest housing, designed to integrate with the patient table inlay in a manner similar to the HNU (Fig 1). By utilizing a biconical, conformal 2D array-shape, the coil layout achieves circumferential coverage of the neck, extending from the skull base to the T1–T2 thoracic segments, with a particular focus on the lateral and posterior cervical regions. This allows the coil to conform closely to the complex contours of this anatomic area. Preliminary work has shown that the FACE provides superior SNR as compared with a rigid 21-channel HNU (Fig 1).
Comparison of SNR maps overlaid on sagittal DESS images of the neck in the same subject. The FACE coil (A) with flexible mask and a rigid posterior head-rest housing, which demonstrates superior SNR in the posterolateral neck regions (B) compared with the conventional HNU coil (C). The brackets in panels (B) and (C) indicate the imaging target regions of occipital nerves.
MR Neurography Protocol
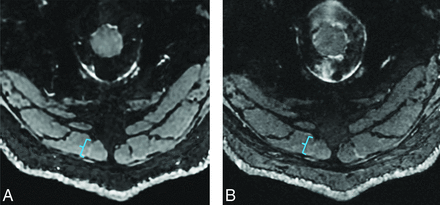
A coronal 3D DESS sequence with the 2 echoes separately reconstructed was performed within 4.5 minutes (TE = 5/9 ms; TR = 15 ms; acquired matrix = 256 × 256 to 316 × 316; FOV = 20.0 × 20.0 cm; slice thickness = 0.6–0.8 mm; receiver bandwidth (RBW) = 139.50–162.7 Hz/pixel; parallel imaging factors = 2 × 2). High, isotropic spatial resolutions (0.6–0.8 mm) were acquired with DESS, adapted to the patient’s body habitus. The 3D DESS sequence was initially obtained at 0.8 mm3 resolution. During real-time monitoring, for some cases the sequence was repeated at 0.6 or 0.7 mm3 resolution to further delineate the anatomy and any associated pathology at the radiologist’s discretion (Fig 2). DESS acquisitions were also reconstructed by using a 3D prototype of a deep learning reconstruction algorithm (AIR Recon DL, GE Healthcare) to enhance SNR and edge sharpness.9 A geometric combination of the 2 echoes was used to further enhance SNR while retaining T2-weighting.9
Comparison of different spatial resolutions in a 36-year-old man with chronic occipital neuralgia. Axial DESS images with 0.8 × 0.8 × 0.8 mm (A) and 0.6 × 0.6 × 0.6 mm (B) resolution show that while anatomic detail is sharper in (B), the right occipital nerve traversing the semispinalis capitis muscle (highlighted by brackets) appears subtly more conspicuous than in (A).
The MR neurography protocol used in this study also included axial and coronal 2D intermediate-weighted FSE (parameters: TE = 25–30 ms; TR = 3000–5000 ms; echo-train length = 16; acquired matrix = 512 × 352; FOV = 16–20 × 16–20 cm; slice thickness = 2–2.5 mm; RBW =244 Hz/pixel) and axial T2-weighted Dixon FSE (parameters: TE = 85 ms; TR = 3000–4500 ms; echo-train length = 15; acquired matrix = 320 × 224; FOV = 16 × 16 cm; slice thickness = 2 mm; RBW = 244.1 Hz/pixel). Furthermore, a 1-mm, isotropic zero-echo time (ZTE) (parameters: TE = 0 ms; TR = 500 ms; acquired matrix = 224 × 224; FOV = 24 × 24 cm; slice thickness = 1 mm; RBW = 488.28 Hz/pixel) sequence was acquired to provide visualization of bony landmarks relative to nerves for aiding in surgical planning or other interventions (Fig 3).
Fused rendering of 3D MR neurography and ZTE images in a 45-year-old man, performed for protocol optimization. A–C, Curved multiplanar reconstruction images from a 0.6 mm isotropic DESS sequence of the (A) GON (arrows), (B) LON (arrows), and (C) TON (arrows). D, Sagittal 1-mm isotropic ZTE image displays the relevant vertebral body levels. E, Fused-rendering of the DESS and ZTE images demonstrates the spatial relationships of the bilateral GONs (blue arrows), left LON (green arrow), and TONs (orange arrows) at the corresponding vertebral levels.
MR Neurography Interpretation.
All images were reviewed postoperatively and jointly by 2 musculoskeletal radiologists (Y.L. and D.B.S.) with 2 and 9 years’ MR neurography experience, respectively. Both radiologists were blinded to the patient’s history and subsequent surgical findings. Visualization of the GON was evaluated (yes/no) at the following 3 sites similar to previous studies8,12: the posterior edge of the obliquus capitis inferior muscle, the entry point to the semispinalis capitis muscle, and immediately deep to the trapezius muscle fascia. Visualization of the LON was also recorded (yes/no) at 3 sites: its emergence from the surface of the levator scapulae muscle, the posterior edge of the sternocleidomastoid muscle, and the level of the occipital protuberance. Visualization of the TON was recorded (yes/no) at 2 sites, from deep and superficial to the semispinalis muscle.
CASE SERIES RESULTS
Thirteen consecutive patients (8 women) with a median age of 52 years (range 35–73) and clinically suspected occipital neuralgia underwent neck MR neurography. Demographic and clinical characteristics of the patients, along with their MR neurography findings and surgical outcomes, are summarized in the Supplemental Data. The median time from symptom onset to imaging was 70 months (range: 7–399 months). Of the 13 patients, 7 had a history of prior surgery, including 3 with prior cervical spine surgery and 4 with prior occipital nerve decompression.
On MR neurography, all segments of GONs were visualized to their superficial course above the trapezius muscle fascia, except in 3 patients with neuroma findings, which are described subsequently. However, segments of LONs beyond the posterior edge of the sternocleidomastoid muscle were not visualized in 5 patients (2 unilateral, 3 bilateral). Additionally, TON segments superficial to the semispinalis capitis muscle were not visualized bilaterally in 3 patients. Notably, in 1 of these patients, bilateral LON and TON were obscured by instrumentation from prior cervical spine surgery.
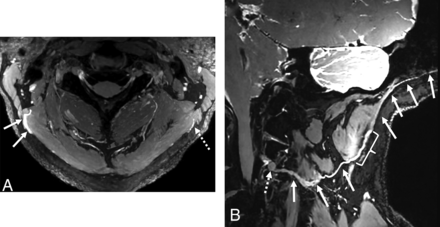
In patients with prior surgery, MR neurography identified occipital terminal neuromas, defined as the nerve terminating in a focally enlarged mass, in 3 patients (1 after cervical spine surgery and 2 after occipital nerve decompression). These neuromas were located deep to the semispinalis capitis muscle and in proximity to prior surgical incisions. Unilateral GON neuromas were found in 2 patients, while neuromas of both the GON and TON were seen in 1 patient (Fig 4). All neuromas were surgically confirmed and resected, except for 1 unilateral GON neuroma, which the surgeon deemed not directly related to the patient’s symptoms. In another case with prior cervical spine surgery, while the GON demonstrated normal signal intensity, arthrosis with osteophyte formation at the right C1–C2 lateral mass articulation resulting in severe compression of the C2 DRG was deemed the most likely etiology of the patient’s neuralgia.
A 39-year-old woman with a history of left GON decompression. Curved multiplanar reconstruction images (A and B) from a 0.6-mm isotropic DESS sequence demonstrate terminal neuromas of both the left GON (solid arrows, A) and left TON (solid arrows, B). The GON neuroma is located deep to the semispinalis capitis muscle and prior surgical incisions (dashed arrows, A), while the TON neuroma is situated adjacent to a focus of susceptibility artifact (dashed arrows, B). Surrounding muscles are labeled for anatomic reference.
Among the 6 patients without antecedent surgery, MR neurography in 1 patient revealed an enlarged and hyperintense right LON (Fig 5), consistent with the patient’s right occipital neuralgia. The asymptomatic, contralateral LON appeared normal.
A 68-year-old woman with a 2-year history of right occipital neuralgia. A, Axial T2-weighted 2D maximum intensity projection Dixon image demonstrates short segment signal hyperintensity (solid arrows) of the right LON as it traverses between the sternocleidomastoid and splenius capitis muscles. Note the normal size and signal intensity of the left LON (dashed arrow). B, Curved multiplanar reconstruction image from a 0.7-mm isotropic DESS acquisition reveals the course of the right LON from the subcutaneous fat overlying the scalp to the C2 dorsal root ganglion (dashed arrow) and confirms the segmental enlargement and hyperintensity (bracket) seen on the 2D Dixon image (A).
DISCUSSION
This study describes the use of a prototype, highly flexible array coil, combined with a high-resolution protocol, for 3D MR neurography of the occipital nerves. Together, these techniques were used to evaluate potential surgical candidates in patients experiencing chronic occipital neuralgia.
A previous study of occipital MR neurography demonstrated the efficacy of a 0.9-mm-isotropic 3D PSIF, a gradient-echo sequence with contrast analogous to the second echo of DESS, to detect increased signal intensity and size alterations of the GON in patients with unilateral, occipital neuralgia.13 Another study utilizing DESS (approximately 0.5 × 0.8 × 0.9 mm spatial resolution) demonstrated its effectiveness in defining the GON posterior to the trapezius fascia and the LON at the level of the occipital protuberance, albeit in patients with suspected salivary gland lesions, not occipital neuralgia.8 Given the small size of the native occipital nerves (∼5 mm in diameter),1,14 we instead targeted an approach utilizing a 3D DESS sequence, with isotropic resolution as high as 0.6 mm, to increase the confidence of localizing neuromas in subjects with prior decompression surgery. Additionally, we evaluated the visualization of the TON, including a case of TON neuroma within our cohort, which to our knowledge is rarely reported on MR neurography. Previous findings by using the FACE coil demonstrated suitability of the coil for cervical spine imaging as well.11 This functionality is particularly beneficial in patients with occipital neuralgia, as arthrosis at the C1–C2 lateral mass articulation is known to result in compression of the C2 DRG,15 and was the likely cause for symptoms in 1 of the patients of this case series.
This study was limited by its small sample size. Further studies with larger cohorts and robust study designs are necessary to confirm the efficacy of the FACE coil in diagnosing occipital neuralgia. Furthermore, this study did not include comparisons of image quality delineating the effects of improved SNR because of the deep learning and geometric image combination reconstructions applied to the DESS sequence. However, the comparisons of the effects from both deep learning and geometric image combination effects had previously been comprehensively demonstrated in the brachial plexus and lumbosacral plexus.9,16 Another limitation was that no systematic, quantitative comparisons were made between the signal intensity and size of abnormal and normal occipital nerve segments, primarily due to the limited number of positive findings in our cohort. Therefore, the efficacy for using the FACE coil on diagnostic interpretability in these nerves (ie, signal and morphology) remains to be determined, because there are few studies to noninvasively establish normative values for signal intensity and size in MR neurography.7
CONCLUSIONS
The 23-channel FACE coil, in combination with advanced imaging reconstruction methods, allowed excellent visualization of the GON and neuromas in all patients with occipital neuralgia, with or without a history of preceding cervical surgery, and the LON and TON in most patients at high spatial resolution (0.6–0.8 mm isotropic). Additional research with larger cohorts is required to validate these results and further assess its clinical utility.
Acknowledgments
The authors thank the HSS Innovation Institute for funding this work. The authors also thank Fraser Robb, PhD, and Jana Vincent, PhD, for performing safety testing of the coil, and Yan Wen, PhD, for his technical support.
Footnotes
This work was partially funded by the HSS Innovations Department.
Indicates article with online supplemental data.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- Received August 25, 2024.
- Accepted after revision November 16, 2024.
- © 2025 by American Journal of Neuroradiology