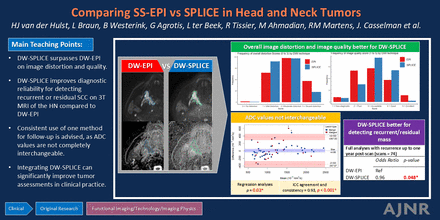
Graphical Abstract
Abstract
BACKGROUND AND PURPOSE: DWI using single-shot echo-planar imaging (DWI-EPI) is susceptible to distortions around air-filled cavities and dental fillings, typical for the head and neck area. Non-EPI, split acquisition of fast spin-echo signals for diffusion imaging (DW-SPLICE) could reduce these distortions and enhance image quality, thereby potentially improving recurrence assessment in squamous cell carcinoma (SCC) of the head and neck region. This study evaluated whether DW-SPLICE is a viable alternative to DWI-EPI through quantitative and qualitative analyses.
MATERIALS AND METHODS: The DW-SPLICE sequence was incorporated into the standard 3T head and neck MRI protocol with DWI-EPI. Retrospective analysis was conducted on 2 subgroups: first benign or malignant lesions, and second, posttreatment SCC recurrence. In both subgroups, image quality and distortion were scored by 2 independent radiologists, blinded to the DWI technique and evaluated using mixed-effect linear models. Lesion ADC values were assessed with interclass correlation and Bland-Altman analyses. The delineation geometric similarity of DWI to T1-weighted postcontrast MRI was evaluated using the DSC before and after registration. Recurrence in posttreatment SCC scans was evaluated by the same 2 radiologists blinded to the DWI technique. Recurrence detection rates were then compared between DW-SPLICE and DWI-EPI using mixed logistic regression at 6 months and 1 year postscan follow-up data.
RESULTS: From August 2020 to January 2022, fifty-five benign or malignant lesion scans (55 patients) and 74 posttreatment SCC scans (66 patients) were analyzed. DW-SPLICE scored better on image quality and showed less overall distortion than DWI-EPI (0.04<P < .001). There was high ADC measurement reliability (intraclsss correlation coefficient = 0.93, P < .001), though a proportional bias was also observed (β = 0.11, P = .03), indicating that the bias increases as ADC values increase. DW-SPLICE exhibited greater geometric similarity to T1WI with gadolinium contrast before registration (DSC 0.63 versus 0.47, P < .001) and outperformed DWI-EPI by more accurately identifying recurrences after 1 year (OR = 0.96, P = .05) but not after 6 months (OR = 0.72, P = .13).
CONCLUSIONS: DW-SPLICE surpasses DWI-EPI on image distortion and quality and improves diagnostic reliability for detecting recurrent or residual SCC on 3T MRI of the head and neck. Consistent use of 1 method for follow-up is advised, because ADC values are not completely interchangeable. Integrating DW-SPLICE can significantly improve tumor assessments in clinical practice.
ABBREVIATIONS:
- DSC
- Dice similarity coefficient
- DW-MS-EPI
- diffusion-weighted multishot echo-planar imaging
- DW-SPLICE
- diffusion-weighted split acquisition of fast spin-echo signal for diffusion imaging
- DW-TSE
- diffusion-weighted TSE
- HN
- head and neck
- ICC
- intraclass correlation coefficient
- SCC
- squamous cell carcinoma
- SENSE
- sensitivity encoding
- T1WIc
- T1-weighted imaging with gadolinium contrast
SUMMARY
PREVIOUS LITERATURE:
DWI using DWI-EPI is known for distortions around air-filled cavities and dental fillings in the head and neck region. DW-SPLICE was proposed to reduce these distortions and improve image quality. Previous studies have suggested potential benefits but lacked comprehensive evaluation.
KEY FINDINGS:
DW-SPLICE showed superior image quality, less distortion, and higher geometric similarity to T1WI with gadolinium contrast MRI than DWI-EPI at 3T. DW-SPLICE also more accurately identified recurrences after 1 year but not after 6 months.
KNOWLEDGE ADVANCEMENT:
This study demonstrates that DW-SPLICE significantly enhances diagnostic reliability for detecting recurrent or residual SCC on head and neck MRI, suggesting its potential for integration into clinical practice on 3T machines, though more research is needed at other field strengths.
DWI, as a functional sequence of MR imaging, excels in differentiating posttreatment effects from tumor recurrence compared with anatomic MR-imaging.1 The sensitivity of DWI to local water mobility on a microstructural level allows it to be less affected by postradiotherapy effects and inflammation, making it irreplaceable for finding and monitoring cancers such as squamous cell carcinoma (SCC) in the head and neck (HN) region.2,3
DWI in oncologic HN protocols is traditionally performed using the single-shot echo-planar imaging (DWI-EPI) technique due to its relatively fast readout and good SNRs. However, DWI-EPI is highly sensitive to susceptibility variations, mainly arising from air-tissue interfaces and metal, leading to considerable artifacts such as geometric distortions and signal voids and accumulations.4,5 This sensitivity is especially a problem for the HN area, considering the air-filled cavities, dental fillings, and reconstruction materials in this anatomic region.
Alternative DWI techniques like multishot echo-planar imaging (DW-MS-EPI) and diffusion-weighted TSE (DW-TSE) have been explored to address these challenges.4
DW-TSE, known for its efficacy in detecting middle ear cholesteatoma,6 has shown promise in HN oncology by differentiating between benign and malignant lesions on the basis of the ADC.7⇓⇓⇓-11 However, adverse results have also been reported, indicating that DW-TSE has limited value in predicting malignancy.12 Given that many studies on DW-TSE have been conducted at suboptimal conditions such as a low magnetic field strength (1.5T)7,9,11,12 or small sample sizes7,8,11 and have not yet evaluated its clinical effectiveness in detecting HN SCC recurrence, further investigation is warranted.
DWI, in general, must be scanned under Carr Purcell Meiboom Gill conditions (https://www.sciencedirect.com/topics/chemistry/carr-purcell-meiboom-gill-sequence), dictating the phase relations and timing in the sequence. By incorporating the diffusion gradients for a DW-TSE sequence in combination with the longer scanning time compared with DWI-EPI, the chance of Carr Purcell Meiboom Gill conditions being violated due to subject motion is high.4 Motion causes phase errors and unstable echo-trains and can lead to signal loss or even signal voids in DW-TSE.5 Modified TSE methods like split acquisition of fast spin-echo signal for diffusion imaging (SPLICE) have been introduced to mitigate this issue.13 DW-SPLICE separates the interfering echo contributions by independently acquiring and reconstructing each echo parity, and afterward, their signal magnitudes are combined. This technique offers less sensitivity to susceptibility and motion artifacts, albeit with a trade-off of a lower SNR compared with DWI-EPI.4,13 Sequence diagrams of the DW-SPLICE and the phase behavior of several echo pathways have been previously visualized by Schick.13 This alternative TSE-based technique has shown the potential for abdominal and HN region MRI and MR-LINAC (MD Anderson Cancer Center) for improving DWI quality by mitigating artifacts.4,10,11,14
The aim of this study was to evaluate whether DW-SPLICE is a better choice than DWI-EPI for lesion and recurrence assessment in the HN region. The analyses will focus on quantitative differences in measured ADC values and the geometric similarity of both techniques compared with anatomic MRI. We also qualitatively evaluate differences through scoring image quality and distortion. Additionally, this study will examine the use of DWI-EPI and DW-SPLICE to identify residual or recurrent tumor in posttreatment HN SCC scans, comparing the results with 1 year of clinical follow-up data.
MATERIALS AND METHODS
Population and Subgroups
In this single-center study at the Netherlands National Cancer Institute, HN MRI scans performed on two 3T MRI scanners between August 2020 to January 2022 were analyzed. An additional DW-SPLICE sequence was added to the standard HN protocol in the context of protocol enhancement. The additional DW-SPLICE scan was not performed in cases of accumulating waiting times in the clinics or when a longer table time would be a burden for the patient, as determined by the radiographers. Patients were not preselected on clinical parameters before imaging. Patients were eligible for consecutive inclusion if the additional DW-SPLICE sequence was conducted in addition to the conventional DWI-EPI scan.
To evaluate the different aspects of the DWI techniques, 2 subgroups of MRI scans were retrospectively selected for analysis. The first subgroup, the so-called lesion-assessment subgroup, included MRI scans of both data sets of untreated benign or malignant lesions or MRI scans of a recurrent lesion after stand-alone surgery. Lesions needed to be visible on at least a b = 1000 image or an ADC map of both the DWI-EPI and the DW-SPLICE scans. A minimal lesion volume of 0.5 cm3 was required for analyses. The second subgroup, the so-called SCC recurrence assessment subgroup, included posttreatment MRI scans of SCC in the HN region. All applicable posttreatment scans were included, regardless of the time between the imaging and the treatment or the treatment type.
The available follow-up data of the patients were organized in accordance with the standard clinical checkup schedule, consisting of clinical examinations including a flexible laryngoscopy every 3 months; additional scans or biopsies were acquired when indicated. Actual recurrence is measured at 6 months and 1 year after the assessed MRI scan was performed.
The Standards for Reporting Diagnostic accuracy studies (STARD)15 methodology has been followed when applicable. An overview of the STARD checklist and the application can be found in the Supplemental Data.
MR Imaging Acquisition
The MR examinations were acquired on a 3T Achieva dStream and a 3T Ingenia system from the same vendor (Philips Healthcare, software Version 5.6.1) and conducted with a dedicated 20-channel HN coil. Aside from the DWI scans, the standard HN protocol consisted of a STIR, a T1-weighted scan with and without a gadolinium contrast agent (T1WI and T1WIc), and an isotropic 3D T1-weighted scan postcontrast. The DWI-EPI had a TE of 67.2–75.8 ms, a TR of 2840.8–6525.9 ms over the 2 scanners, with a 4.0-mm slice thickness and scan time ranging from 110.8 to 254.5 seconds. The DW-SPLICE had a TE/TR of 62.4–71.6 ms/4250.4–5659.3 ms over the 2 scanners, 4.0-mm slice thickness, and a scan time ranging from 187.0 to 249.0 seconds. B-values of 0, 200, and 1000 s/mm2 were collected for both DWI techniques. ADC maps were calculated using all available b-values. Full imaging parameters of the DWI and T1WIc scans can be found in Table 1.
MRI scanning parameters overviewa
Data Preparation
The DWI-EPI and DW-SPLICE scans of each patient were separated, randomized, and blinded for DWI technique and patient-specific information within their subgroup, creating double the amount of cases compared with the amount of patients. For every DWI case, b = 0, b = 1000, ADC, and T1WIc were available for the readers. Within the lesion-assessment subgroup, the T1WIc scans received separate randomization and blinding for delineation, with readers additionally having access to corresponding STIR images.
Delineation and Registration
All scans in the lesion-assessment subgroup were delineated independently on the blinded DWI b = 1000 scans and on the T1WIc scans by a physician researcher (H.J.v.d.H.) and supervised by a radiologist (G.A.) with 5 years of radiology experience. ROIs were manually placed on the entire tumor volume using 3D-Slicer software (Version 4.10.2; http://www.slicer.org). ROIs were placed in accordance with areas of high signal intensity at b = 1000, or, when not discernible at b = 1000, on high- or low-intensity areas on the ADC map. When possible, ROIs are chosen for their high signal intensity on b = 1000 coupled with low signal intensity on ADC, to depict diffusion restriction.16 Large cystic or necrotic areas were excluded if additional, noncystic or non-necrotic, tissue could be identified, ensuring that these areas did not impact the ADC measurement. An exception was applied to lesions that were exclusively cystic in nature. ROIs delineated on the DWI b = 1000 were used for the extraction of the ADC values. Raters were blinded to the lesion type during delineation.
To determine the geometric similarity of the DWI delineations to anatomic MRI, DWI-EPI, and DW-SPLICE, we registered scans to their corresponding T1WIc scan using Advanced Normalization Tools (ANTs) (Version 0.0.7; http://stnava.github.io/ANTs/). After registration, the same transformation was applied to the DWI delineations. The volume of the DWI-EPI and DW-SPLICE delineation that overlaps with the T1WIc delineation was measured before and after registration.
Qualitative Evaluation
All data from both subgroups were independently scored on image quality and distortion by 2 HN radiologists (L.B. and B.W.) with 5 and 3 years of experience, using a standardized score form. Image quality was defined as the following: 1 = nondiagnostic quality, 2 = poor quality, 3 = acceptable quality, 4 = good quality, 5 = excellent quality.17 Distortion was scored on the overall image and also specifically on the lesion location for all cases within the lesion-assessment subgroup. Distortion was defined as the following: 0 = no image distortion, 1 = mild image distortion, 2 = moderate image distortion, 3 = severe image distortion.18 Cases within the SCC recurrence assessment subgroups were additionally scored for the presence of recurrent or residual disease using a binary scoring system, defined as 0 = not suspect for recurrence or residual disease and 1 = suspect for recurrence or residual disease. Consistency in scoring among raters was ensured through a kick-off meeting, followed by an evaluation after the first 5 cases. Full score forms can be found in Supplemental Data.
Statistical Analyses
R statistics (Version 4.3.3; http://www.r-project.org/) was used for all analyses. A P value < .05 was considered statistically significant.
The association between the qualitatively scored image quality and distortion of both the DWI types (EPI or SPLICE) was assessed using a linear mixed-effect model. This model was used to incorporate the dependence existing between observations in the data set as a result of the 2 independent raters and the potential scoring link between the DWI of the same case. Therefore, 2 random intercepts were used in the model at the 2 fixed rater level (2 radiologists) and the MRI scan pair level (DW-SPLICE and DWI-EPI). Additionally, the interrater variability was assessed using a quadratically-weighted Cohen κ.
ADC value differences between DWI-EPI and DW-SPLICE were analyzed using the type 3 intraclass correlation coefficient (ICC) for agreement and consistency and Bland-Altman regression to detect any systematic bias or significant discrepancies. The geometric similarity of the DWI relative to the T1WIc was evaluated using the Dice Similarity Coefficient (DSC), Recall score and Precision score. The DSC evaluates how closely the areas delineated on DWI and T1WIc overlap. The Recall score focusses on the effectiveness of identifying T1WIc areas by measuring the proportion of T1WIc delineation correctly captured within the overlapping area with DWI delineation. The Precision score evaluates the precision of DWI delineation by quantifying the proportion of DWI delineation that does not overlap with T1WIc delineation. Comparisons of the scores between the DWI techniques were made using Wilcoxon signed-rank tests. Formulas of the DSC, Recall score and Precision score are defined in the Supplemental Data.
In the SCC recurrence subgroup, the interrater variability was assessed using the Cohen κ. The recurrence score was assessed using mixed logistic regression to adjust for the inter-/intrareader variability, using the binary recurrence score and the actual outcome data after both 6 months and 1 year postscan follow-up. The model uses the agreement between the scorers and the actual outcome. The random intercepts applied in these analyses were the same as those for the image quality and distortion analyses, the 2 fixed rater level (2 radiologists), and the MRI scan pair level (DW-SPLICE and DWI-EPI). Additionally, the diagnostic accuracy and receiver-operating characteristic curve were calculated after both 6 months and 1 year postscan follow-up as a supplementary test. While less suitable to the data due to the lack of incorporating the potential intercepts, these tests provide an additional insight into the data.
RESULTS
Between August 2020 and January 2022, a total of 396 MR scans targeting the HN area were completed on the selected 3T scanners in the Netherlands National Cancer Institute. In 214 of these scans, the additional DW-SPLICE sequence was added to the scan protocol. Fifty-five of these 214 scans depicted untreated benign or malignant lesions of the HN or recurrent masses after stand-alone surgical treatment and were included in the lesion-assessment subgroup. Additionally, 74 of these 214 scans depicted posttreatment SCC of the HN region. These scans were selected for the SCC recurrence subgroup. See Fig 1 for the full flow diagram and exclusion criteria. Baseline characteristics of all subgroups can be found in the Supplemental Data.
Patient inclusion and flow diagram. Figure illustrating the patient inclusion process (overall n = 121) (lesion assessment subgroup n = 55, SCC recurrence subgroup n = 66), detailing reasons for exclusion and outlining the inclusion criteria and sample sizes for the separately analyzed subgroups. n indicates the number of patients in the subcohort.
Image Quality and Distortion Assessment
All 129 scans from 121 patients in the lesion and SCC recurrence subgroups were scored for image quality and distortion. Baseline characteristics can be found in the Supplemental Data. Total frequencies of scores on the (estimated) tumor distortion, the overall image distortion, and image quality are depicted in Fig 2. DW-SPLICE scans were more frequently rated as having “no-to-mild distortion” compared with DWI-EPI scans, both for tumor-specific (74.0% versus 67.6%) and overall distortion (43.3% versus 31.6%). In contrast, DWI-EPI scans more often received “moderate-to-severe distortion” ratings (tumor-specific: 32.3%, overall: 68.4%) compared with DW-SPLICE scans (tumor-specific: 26.0%, overall: 56.7%). In terms of image quality, DW-SPLICE was more frequently rated as “good” or “acceptable” (77.0% versus 56.6%), whereas DWI-EPI scans were more commonly rated at the extremes of “poor” (31.9% versus 17.9%) or “excellent” (6.8% versus 1.2%).
Distribution of rater scores. Frequency distribution of rater scores for tumor distortion (0–3) (n = 55, 55 scans) (A), overall distortion (0–3) (n = 121, 129 scans) (B), and image quality (1–5) (n = 121, 129 scans) (C), as evaluated by the 2 independent radiologists. Lower scores (0 or 1) denote less distortion for A and B, while higher scores (4–5) indicate better image quality for C.
There was limited-but-acceptable agreement between raters for the tumor-specific distortion score in DWI-EPI and DW-SPLICE, as well as for the overall distortion and image-quality score in DW-SPLICE (Table 2). However, for the DWI-EPI overall distortion and image-quality scores, no agreement was seen when analyzing the weighted κ between the raters (Table 2). Differences in rater-specific scoring are highlighted in the Supplemental Data.
Results of the quadratically weighted κ and mixed-effect linear modelsa
Within the mixed-effect linear models, which accounted for rater variability and the DWI pairs, no significant effect of the DWI technique on the tumor-specific distortion score was observed (Table 2). Yet, there was a significant effect of the DWI technique on the overall distortion score, with DW-SPLICE having an, on average, lower score and thus less distortion compared with DWI-EPI (Table 2). Additionally, DW-SPLICE showed a significantly higher score for image quality, indicating better image quality compared with DWI-EPI (Table 2).
Lesion Assessment
Of the 55 scans, 36 scans from 36 unique patients showed ≥1 DWI-visible lesion with a volume of >0.5 cm3. Baseline characteristics from this analyzed subgroup and the 56 included lesions (39 benign lesions, 17 malignant lesions) can be found in the Supplemental Data. An example of DWI-EPI and DW-SPLICE delineation is shown in Fig 3.
Comparative delineation on DWI-EPI and DW-SPLICE images. Example of DWI-EPI delineation shown on b = 1000 (A) and ADC images (B), compared with the same case delineated by using DW-SPLICE displayed on b = 1000 (C) and ADC images (D). The matching T1WIc delineation is shown in E. DW-SPLICE images exhibit lower contrast compared with DWI-EPI, particularly in the ADC map, due to a lower SNR. In contrast, DW-SPLICE offers visually higher geometric similarity to T1WIc compared with the DWI-EPI. Please note that considering the lower SNR, a low threshold for masking was used for the DW-SPLICE to avoid image gaps, resulting in more noise around the image.
The ICC analysis showed the high reliability of the DW-SPLICE and DWI-EPI ADC values across all included lesions and the benign or malignant lesion subanalyses with agreement and consistency of 0.85–0.96 (Table 3).
ICC results of the lesion ADC valuesa
Bland-Altman plots and the regression analyses over all lesions and the benign lesion subanalyses showed a proportional bias in which the difference between DWI-EPI and DW-SPLICE ADC increases for higher values (Table 4, Fig 4A, -B). This effect was not seen when only the malignant lesions were analyzed (Table 4, Fig 4C).
Bland-Altman plots of the lesion ADC values. Visual ADC value difference between the DWI-EPI (reference) and the DW-SPLICE for all lesions (A), only the benign lesions (B), and only the malignant lesions (C). Lesion X: a papillary thyroid carcinoma, located low in the neck, not completely depicted on both DWI’s. Lesion Y: a glomus tumor located low in the neck.
ICC Bland-Altman analysis of the lesion ADC valuesa
Compared with the anatomic T1WIc delineations before registration, the DW-SPLICE delineations demonstrate significantly better geometric similarity with higher DSC scores (0.63 versus 0.47, P < .001), better recall (0.63 versus 0.45, P < .001), and greater precision (0.67 versus 0.52, P < .001) compared with DWI-EPI delineations. After registration, these differences dissipate, and the scores equalize. See the Supplemental Data for the full results. Figure 3 depicts an example of the T1WIc delineation and both DWI delineations.
SCC Recurrence Assessment
Of the 66 patients included in the SCC recurrence assessment subgroup, 43.9% had oropharyngeal SCC and 57.6% received systemic therapy such as radiation and/or chemotherapy. Of the 74 corresponding scans, 52.7% were performed within 6 months after initial treatment. Complete baseline and treatment characteristics can be found in the Supplemental Data. There was an overall mean follow-up of 1.5 years postscan.
DW-SPLICE recurrence scoring showed a better, though not statistically significant, association with actual recurrence at 6 months (Table 5) and a statistically significant improvement at 1 year compared with DWI-EPI (Table 5). A subset analysis focusing on scans with confirmed recurrence further confirmed the superiority of DW-SPLICE in accurately identifying actual recurrence (Table 5). A significant agreement between the 2 reviewers’ scores was seen for DWI-EPI and DW-SPLICE (Table 5).
Cohen κ and mixed linear regression results for recurrence or residual mass at 6 months and at 1 year
DW-SPLICE demonstrated a higher sensitivity (47.9% versus 27.1%) and similar specificity (93.0% versus 94.0%) in diagnosis recurrence at 1-year follow-up compared with DWI-EPI. These and other diagnostic accuracy measurements with receiver operating characteristic curve and cross-tabular data can be found in the Supplemental Data.
DISCUSSION
This study offers a comprehensive evaluation of 2 DWI techniques (DWI-EPI and DW-SPLICE), by examining their differences in measured ADC values, geometric similarity to T1WIc MRI, and qualitatively scored distortion and image quality. Additionally, we evaluated their effectiveness in detecting recurrences of SCC in the HN region. Our findings indicate that DW-SPLICE surpasses DWI-EPI on 3T MRI in several areas, including reduced overall image distortion, higher image quality, and diagnostic accuracy for detecting recurrent and residual SCC. Thus, integrating DW-SPLICE into standard HN MRI protocols can significantly improve tumor assessment.
DW-SPLICE scans showed significantly better image quality and less overall distortion compared with DWI-EPI scans (Table 2). Although the tumor-specific distortion score was also better for DW-SPLICE, this difference was not statistically significant, possibly due to the smaller number of patients evaluated for this metric. These findings are in line with the results of Hirata et al17 and Fu et al,19 showing that DW-TSE sequences provide qualitatively better image quality and fewer artifacts than DWI-EPI in the oral and ocular regions, respectively.
Our mixed-effect linear models, which account for the random effects of the raters, show overall better scores for DW-SPLICE. However, a potential rater bias, as shown by the lack of agreement in overall distortion and image-quality weighted κ scores of DWI-EPI. shown in Table 2 and further visualized in the Supplemental Data, needs to be addressed. Several standardization and training measures such as a kick-off meeting and an evaluation after the first cases were conducted, yet more training may have been necessary or the score should have been simplified. Tumor-specific κ scores improved after simplifying the scoring into 2 categories: “no distortion” (including none and mild distortion) and “distortion” (encompassing moderate-to-severe distortion). Possibly, the time between the standardization measures and the actual evaluations also influenced the results, because disagreements were observed only in the overall distortion and quality scores and less in the tumor-specific distortion score, which was rated only in the first subgroup (Supplemental Data). Unlike DWI-EPI, DW-SPLICE did not encounter the same rater disagreement. This discrepancy could stem from the raters’ limited familiarity with DW-SPLICE, reducing unconscious biases and, thus, improving adherence to the original scoring guidelines. However, this could also indicate that DW-SPLICE may provide more consistent scores across raters due to better readability and just less reliance on experience with the sequence.
Because DWI is used as a functional technique, reproducibility of the already-established ADC values of DWI-EPI is of high importance.17,20 Within this study, a high degree of ICC reliability is seen between the ADC values of the 2 diffusion-weighted techniques. Yet, a proportional bias was observed in ADC measurements of DW-SPLICE compared with DWI-EPI within the overall and benign lesion analyses (Table 3 and Fig 4). This indicates that DW-SPLICE ADC values of these groups are higher than the DWI-EPI ADC values, with this difference increasing for higher ADC values. Previous tests by Schakel et al4 that compared ADC values of DW-SPLICE and DWI-EPI using ice water phantoms did corroborate the established literature values. Additionally, a Bland-Altman analyses of Panyarak et al10 did not find a proportional bias in the salivary glands or lesions in the HN region of DW-TSE compared with DWI-EPI. In our study, variations in delineation might have contributed to the observed differences in ADC values. Delineations for DWI-EPI and DW-SPLICE were performed independently and blinded, with matching done retrospectively, which could have led to slight inconsistencies. This issue is particularly pronounced in benign lesions, which are harder to delineate due to variations in ADC or b = 1000 contrast and the variety in locations. This is strengthened by the largest discrepancies being observed in lesions located lower in the neck, with less signal from the coil, or that were incompletely depicted (Fig 4A, -B). Moreover, the presence of 2 lesions visible on DWI-EPI but not on DW-SPLICE and 3 lesions visible on DW-SPLICE but not on DWI-EPI indicate some variation in lesion visibility between the DWI techniques.
Overall, this issue suggests that ADC values derived from these 2 techniques should not be considered interchangeable. Instead, a single technique should be consistently used throughout the staging and follow-up timeline.
While DWI is used as a functional imaging technique relying on a perceived ADC value, enhancing geometric accuracy is essential for accurately detecting small lesions, particularly in areas with variable magnetic susceptibility. Reducing susceptibility-induced distortion common in DWI-EPI and improving geometric similarity helps prevent small lesions from being missed or misidentified as artifacts.1,4,5 To measure the level of distortion across the 2 DWI techniques, we compared the overlap between the DWI delineations and the more anatomically accurate T1WIc delineations. Because T1WIc and DWI depict different aspects of lesions, a perfect match was not anticipated. However, we postulated that most of the areas discernible on DWI would fall within the T1WIc delineation. In our data, the DW-SPLICE showed a better delineation match compared with DWI-EPI before registering the image to the T1WIc (Supplemental Data), corroborating the results of Panyarak et al,10 which superimposed their DW-TSE and DWI-EPI on the T2-weighted imaging to calculate a distortion ratio. After registering the DWI to the T1WIc images, this effect dissipates as match percentages equalize, underlining that the same lesion information is likely available in both scans but just hampered by distortion. In clinical radiology practice, where no image registration is applied, radiologists may benefit from using the more accurate DW-SPLICE for locating and assessing lesions.
DWI is beneficial for managing patients with SCC because it is less affected by postradiotherapy effects and inflammation.1,20 Therefore, this study focuses on comparing the diagnostic capabilities of DWI-EPI and DW-SPLICE in detecting recurrent or residual SCC after treatment. Our results indicate that DW-SPLICE was more effective in detecting residual or recurrent SCC at 1 year follow-up after the assessed MRI scan (P = .048). Raters agreed to base their assessments primarily on what was visible on DWI, though the pretreatment tumor location and stage and an additional sequence (T1WIc) were available for a limited clinical context. Raters lacked access to prior and/or baseline scans and full clinical and treatment details, which are typically considered in standard clinical practice. This omission shows that DW-SPLICE potentially is a superior technique to assess response.
To optimize diffusion-weighted image quality, we chose to include only scans obtained on 3T MRI scanners. Clinical MRI scanners with lower field strengths require longer diffusion gradients, which result in higher TE and noisier DWI. This requirement is especially a problem for DW-TSE like DW-SPLICE, which already has a longer acquisition.4,17 Considering that clinical MRI scanners are now increasingly equipped with stronger-gradient hardware, we opted to test the DW-SPLICE under ideal circumstances.3 However, as susceptibility artifacts scale with field strength, scanning at higher field strengths may be less advantageous for DWI-EPI. This possibility suggests that our results might not be as pronounced at lower field strengths.17 Furthermore, as sensitivity encoding (SENSE) was applied to all DWI scans, standard SNR calculation was not feasible because the distribution of noise was not uniform over the scan.21
Other limitations include the retrospective study design, resulting in variations in lesion types, time between scan and treatment, and treatment types. In clinical practice, radiographers may adjust the field of view during scans. Although the dimensions of the DWI-EPI and DW-SPLICE scans are typically linked, in some cases, the field of view of the DWI-EPI scan was altered without applying the same adjustment to the corresponding DW-SPLICE scan. This step led to more variations in scan duration, resulting in a more varied TR for the DWI-EPI scans. While these TR variations are not expected to affect the ADC,22 the differences in FOV could potentially have a minor impact on how radiologists interpret the data. A prospective comparison of the 2 techniques in a more standardized group of patients would be of interest. The reliability of DW-SPLICE for detecting a recurrent and residual HNSCC mass at a lower field strength should be assessed in future work.
Aside from the DW-SPLICE, several other DWI methods exist to mitigate the artifacts seen for single-shot DWI-EPI scans, either by using a multishot EPI sequence or by applying the PROPELLER technique for reconstruction.23,24 Other non-EPI techniques such as HASTE likewise show value in discerning cholesteatoma but do not always outperform DWI-EPI for HN SCC assessment.7,25,26 Although DW-SPLICE outperformed the DWI-EPI sequence, a comprehensive comparison with all other alternative techniques would be of interest.
CONCLUSIONS
This study shows that DW-SPLICE outperforms DWI-EPI in reducing image distortion, enhancing image quality, and improving stand-alone diagnostic reliability for detecting recurrent and residual SCC while scanning at a high field strength (3T). However, ADC values from these 2 techniques are not interchangeable, and consistent use of a single technique for follow-up is advised. Our data support the integration of DW-SPLICE into clinical practice at 3T, because this could improve tumor status assessments. Future work should evaluate the value of DW-SPLICE at a lower field strength for full clinical integration.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- Received June 19, 2024.
- Accepted after revision October 7, 2024.
- © 2025 by American Journal of Neuroradiology